Abstract
Antihistamines are commonly used to treat allergic disease, such as allergic rhinitis, urticaria, and angioedema. Although several previous reports describe hypersensitivity to antihistamines such as cetirizine and hydroxyzine, documented cases of chlorpheniramine hypersensitivity are extremely rare. Here, we report the case of a 45-year-old Korean woman who presented with urticaria after ingesting a cold medication. Over the previous 5 years, she had also experienced a food allergy to crab and shrimp, allergic rhinitis, and repeated urticaria after ingesting cold medication. Provocation with aspirin elicited generalized urticaria. Intravenous chlorpheniramine and methylprednisolone was injected for symptom control, but in fact appeared to aggravate urticaria. A second round of skin and provocation tests for chlorpheniramine and methylprednisolone showed positive results only for chlorpheniramine. She was diagnosed with aspirin intolerance and chlorpheniramine hypersensitivity, and was instructed to avoid these drugs. To date, this is the second of only two cases of chlorpheniramine-induced type I hypersensitivity with aspirin intolerance. Although the relationship between aspirin intolerance and chlorpheniramine-induced type I hypersensitivity is unclear, physicians should be aware of the possibility of urticaria or other allergic reactions in response to antihistamines.
Histamine released from mast cells and basophils plays an important role in the pathophysiology of allergic diseases, such as asthma, allergic rhinitis, urticaria, and anaphylaxis.1,2 Histamine exerts various effects through four histamine receptors. Allergic symptoms, such as itching, pain, vasodilation, hypotension, flushing, bronchoconstriction, and the stimulation of cough receptors, develop mainly through histamine receptor 1 (H1R), making this receptor a major target of allergic medications.1,2 H1R histamine antagonists can be further classified as first- or second-generation agents; whereas a first-generation drug may have a sedative effect, due to an interaction with receptors in the central nervous system, second-generation agents are less lipophilic and are thus less able to cross the blood-brain barrier to induce a sedative effect.1,2 Interestingly, however, antihistamine treatment itself may also elicit an allergic response. Several cases of hypersensitivity reactions against piperazine derivatives such as cetirizine, hydroxyzine, levocetirizine, and loratadine have been reported.3-6 However, hypersensitivity reactions to alkylamine derivatives such as chlorpheniramine maleate are extremely rare, particularly a type I reaction.7,8 To date, only one case of chlorpheniramine-induced type I hypersensitivity with aspirin intolerance has been reported. Here, we report a second case of type I hypersensitivity to chlorpheniramine maleate with aspirin intolerance.
A 45-year-old woman visited the Asthma and Allergy Clinic of Seoul National University at Bundang Hospital seeking treatment for urticaria after taking cold medicine. She reported a 5-year history of urticaria, rash, and most recently mild dyspnea after taking cold medications. The patient indicated that she had never taken aspirin before and could not recall whether she had ever experienced an adverse reaction to acetaminophen. She also suffered from allergic rhinitis and a food allergy to crab and shrimp. Her mother had a history of penicillin allergy, but no other allergic disease. At the time of presentation, the patient had recently consumed medications containing clarithromycin, loxoprofen, acetaminophen, cimetidine, metoclopramide, magnesium oxide, chlorpheniramine, and simethicone. Physical examination showed no specific signs. Blood analyses indicated her leukocyte count to be 22,890×103 cells/µL (neutrophils, 58.6%; lymphocytes, 22.4%; monocytes, 4.9%; eosinophils, 0.3%) and other blood cell counts were within normal ranges. The patient's total IgE level was elevated at 333 IU/mL.
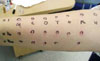
A skin prick test for inhalant allergens (Allergopharma, Reinbek, Germany) showed strong positive responses for Dermatophagoides farinae, Dermatophagoides pteronyssinus, Tyrophagus putrescentiae, and Tetranychus urticae. A methacholine bronchial provocation test elicited no abnormal response. As we suspected aspirin idiosyncrasy, an oral provocation test was performed by administering an oral placebo drug, followed by sequential aspirin administration (25, 50, and 100 mg) at 30-min intervals. Within 30 min of the 100-mg oral dose, urticaria developed at the lips and on both arms and hands, with accompanying eyelid edema. The patient did not complain of dyspnea and her forced expiratory volume in one second (FEV1) did not decrease. We concluded that the provocation test was positive and diagnosed her with aspirin idiosyncrasy. For symptomatic control, we administered intravenous chlorpheniramine at 45.5 mg and methylprednisolone at 40 mg. However, this treatment aggravated urticaria and we subsequently performed another round of skin prick and intradermal tests for chlorpheniramine, methylprednisolone, hydrocortisone, dexamethasone, and triamcinolone. Positive skin prick (3×3 mm) and intradermal (wheal, 7×6 mm; flare, 10×10 mm) responses were observed for chlorpheniramine (Figure), but not methylprednisolone, leading to suspicion of chlorpheniramine hypersensitivity. To obtain a definitive diagnosis, we performed an intramuscular chlorpheniramine provocation test. Briefly, three solutions (0.25 mL normal saline, 0.25 mL chlorpheniramine at 22.75 mg/mL, and 2 mL chlorpheniramine at 22.75 mg/mL) were prepared and then labeled in such a manner so as to blind the administering physician (treatments A, B, and C, respectively). We administered each drug intramuscularly and observed the patient's response every 30 min. No response was observed after the injection of solution A (control); however, the injection of solution B elicited tiny reddish papules on the patient's neck and both arms, and these lesions worsened after injecting solution C. We thus diagnosed the patient with chlorpheniramine hypersensitivity. The patient tolerated fexofenadine before. Skin prick, intradermal, and oral provocation tests for antibiotics such as penicillin and cephalosporins showed no positive result. Provocation tests with acetaminophen and celecoxib were negative. Finally, we diagnosed the patient with aspirin idiosyncrasy and chlorpheniramine hypersensitivity, and recommended her to avoid these two drugs. Subsequently, for the past 3 years, she has not experienced recurrence of the reported symptoms.
Antihistamine hypersensitivity is very unusual, with only a few published reports of hypersensitivity reactions to piperazine derivatives, such as cetirizine, hydroxyzine, and loratadine.3-6 In contrast, hypersensitivity to alkylamine derivatives such as chlorpheniramine is extremely rare,7,8 particularly a type I reaction.9 Recently, in Korea, the case of a 42-year-old male displaying urticaria, angioedema, dyspnea, and hypotension after ingesting chlorpheniramine and cetylpyridium was reported; this patient also suffered from aspirin-intolerant asthma.10 The authors confirmed chlorpheniramine hypersensitivity through both skin tests and oral provocation. Although the ingestion of 0.5 mg chlorpheniramine resulted in urticaria, cetylpyridium provocation elicited no response. The patient tolerated oral provocation with cetirizine, although a skin prick test with the same compound elicited a positive response. This case is notable compared to our case because both are characterized by aspirin intolerance. Aspirin has been known to aggravate food-dependent exercise-induced anaphylaxis.11 However, the relationship between aspirin intolerance and chlorpheniramine hypersensitivity is unclear. This is the second case report of chlorpheniramine hypersensitivity with aspirin intolerance.
Allergic responses to chlorpheniramine in skin prick, intradermal, and oral provocation tests points to IgE-mediated type I hypersensitivity as the mechanism of chlorpheniramine hypersensitivity reaction in this case. In a previous study, a hypersensitive patient showing a positive patch test to cetirizine also reacted positively to patch tests for hydroxyzine and levocetirizine, suggesting cross-reactivity between antihistamines in the same chemical family.4 In one of our own previous studies, cross-reactivity between antihistamines was confirmed using the oral provocation test in a patient with cetirizine hypersensitivity. We observed cross-reactivity between cetirizine and hydroxyzine (piperazine family), but not with levocetirizine (piperazine), loratadine (piperidine), fexofenadine (piperidine), ebastine (piperidine), or chlorpheniramine (alkylamine). These results indicated that piperidine or alkylamine antihistamines may be safe alternative drugs in the case of piperazine hypersensitivity.12 However, for a patient with hypersensitivity to chlorpheniramine, a member of the alkylamine derivatives, it would be better to avoid similar alkylamine derivatives such as dexchlorpheniramine, bromchlorpheniramine, and triprolidine, or to use the piperazine, piperidine, or ethanolamine derivatives with caution. Further study is needed to better understand cross-reactivity within antihistamines.
Here, we described a case of chlorpheniramine-induced type I hypersensitivity with aspirin-induced urticaria. Physicians should be aware of the possibility of urticaria or other allergic responses in response to antihistamines.
Figures and Tables
ACKNOWLEDGMENTS
This study was supported by grants from the Korean Health 21 R&D Projects, Ministry of Health & Welfare and Family Affairs, Republic of Korea (A030001).
Notes
References
1. Holgate ST, Church MK, Lichtenstein LM. Allergy. 2006. 3rd ed. Philadelphia: Mosby Elsevier.
2. Grammer LC, Greenberger PA. Patterson's allergic diseases. 2009. 7th ed. Philadelphia: Lippincott Williams & Wilkins.
3. Inomata N, Tatewaki S, Ikezawa Z. Multiple H1-antihistamine-induced urticaria. J Dermatol. 2009; 36:224–227.
4. Cravo M, Goncalo M, Figueiredo A. Fixed drug eruption to cetirizine with positive lesional patch tests to the three piperazine derivatives. Int J Dermatol. 2007; 46:760–762.
5. Mahajan VK, Sharma NL, Sharma VC. Fixed drug eruption: a novel side-effect of levocetirizine. Int J Dermatol. 2005; 44:796–798.
6. Lew BL, Haw CR, Lee MH. Cutaneous drug eruption from cetirizine and hydroxyzine. J Am Acad Dermatol. 2004; 50:953–956.
7. Cáceres Calle O, Fernández-Benítez M. Allergy to dexchlorpheniramine. Study of a case. Allergol Immunopathol (Madr). 2004; 32:306–309.
8. Demoly P, Messaad D, Benahmed S, Sahla H, Bousquet J. Hypersensitivity to H1-antihistamines. Allergy. 2000; 55:679–680.
9. Thurot-Guillou C, Bourrain JL, Jacquier JP, Beani JC. Anaphylactic reaction to ranitidine and dexchlorpheniramine. Eur J Dermatol. 2007; 17:170–171.
10. Lee SH, Jung HS, Yoon TY, Chang EJ, Kim MK, Kim KS. Allergic reaction to chlorpheniramine in a patient with aspirin-intolerant asthma. Korean J Asthma Allergy Clin Immunol. 2010; 30:55–58.
11. Harada S, Horikawa T, Ashida M, Kamo T, Nishioka E, Ichihashi M. Aspirin enhances the induction of type I allergic symptoms when combined with food and exercise in patients with food-dependent exercise-induced anaphylaxis. Br J Dermatol. 2001; 145:336–339.
12. Chang YS, Kwon HS, Cho SH, Kim YY, Min KU. A case of urticaria induced by both hydroxyzine and cetirizine but not by levocetirizine. Allergy. 2007; 62:819–821.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download