Abstract
Purpose
Food allergies can affect the growth and nutritional status of children with atopic dermatitis (AD). This study was conducted to determine the association between the number of sensitized food allergens and the growth and nutritional status of infants and young children with AD.
Methods
We studied 165 children with AD, aged 5 to 47 months, and who visited the Atopy Clinic of the Seoul Medical Center. We recorded the birth weight, time at which food weaning began, scoring of atopic dermatitis (SCORAD) index, eosinophil counts in peripheral blood, and total serum IgE and specific IgE to six major allergens (egg white, cow's milk, soybean, peanut, wheat, and fish). The height and weight for age and weight for height were converted to z-scores to evaluate their effects on growth and nutritional status. Specific IgE levels ≥0.7 kUA/L, measured via the CAP assay, were considered positive.
Results
As the number of sensitized food allergens increased, the mean z-scores of weight and height for age decreased (P=0.006 and 0.018, respectively). The number directly correlated with the SCORAD index (r=0.308), time at which food weaning began (r=0.332), eosinophil counts in peripheral blood (r=0.266), and total serum IgE (r=0.394). Inverse correlations were observed with the z-scores of weight for age (r=-0.358), height for age (r=-0.278), and weight for height (r=-0.224).
Conclusions
A higher number of sensitized food allergens was associated with negative effects on the growth and nutritional status of infants and young children with AD. Therefore, a thorough evaluation of both growth and nutritional status, combined with adequate patient management, is crucial in pediatric AD patients presenting with numerous sensitized food allergies.
Allergic diseases are multifactorial and induced by a combination of genetic and environmental factors.1,2 Atopic dermatitis (AD) is more strongly related to food allergens in children than in adults.3 In pediatric patients with AD, the major food allergens include egg white, cow's milk, soybean, wheat, and peanut.4 Food allergies manifest various symptoms in the skin, gastrointestinal tract, and airways as a result of adverse responses to a food protein via IgE-mediated or non-IgE-mediated immune mechanisms. Allergic reactions to food present as both acute and chronic inflammation due to cellular responses activated through specific IgE against the food allergen.5
The basic principle in the treatment of food allergies is the avoidance of the allergen. AD associated with food allergy can be successfully controlled with meticulous skin care, drug therapy, and the restriction of foods containing sensitized allergens.6 However, the restriction of food consumption can lead to nutritional deficiency, and thus for normal body growth and the prevention of disease, a nutritional balance must be achieved through the intake of alternative foods.7-9 Additionally, malnutrition owing to food avoidance results in weight loss and damage to the immunomodulatory system, which subsequently exerts a negative effect on the progress, treatment, and prognosis of disease.10 It has been reported that some pediatric AD patients are short,11 and growth retardation such as short height or low body weight has been reported in patients with AD.12 Previous studies have reported the occurrence of nutritional problems related to food allergies, including kwashiorkor,13 rickets,14 and marasmus,15 in infants with AD, following food restriction.
In addition, because they are in an active growth phase, pediatric AD patients with food allergies are at particularly high risk for developing growth retardation and nutritional deficiency; these patients require careful management for growth and nutrition.16 Therefore, this study investigated both growth and nutritional status according to the number of sensitized food allergens in infants and young children with AD.
This study included a total of 165 children between the ages of 5 and 47 months who were diagnosed with AD according to the diagnostic criteria proposed by Hanifin and Rajka17, by pediatricians at our clinic.
For children aged <24 months, the height and weight were measured with the child in a recumbent position, with the shoes off, and with a light top on, using an automated height and weight measuring instrument. For children aged ≥24 months, the height and weight were measured to 0.1 cm with the child in a standing position, with the shoes off, and with a light top on, using an automated height and weight measuring instrument. The normal values of Korean children and adolescents proposed by the Korean Pediatric Society in 2007 were used as reference values. The growth and nutritional status were evaluated using the z-scores of the World Health Organization.18 Weight for age, height for age, and weight for height are expressed as z-scores in standard deviation units. A weight for age, height for age, or weight for height z-score of less than -2 represents moderate to severe undernutrition.19
The eosinophil counts obtained by routine blood tests were used as a biochemical marker. Total serum IgE and specific IgE against sensitized food allergens were measured using an ImmunoCAP assay (Pharmacia, Uppsala, Sweden). A specific IgE level of ≥0.7 kUA/L defined sensitization to egg white, cow's milk, soybean, peanut, wheat, and fish. The number of these six sensitized food allergens was calculated for each patient, and the patients were divided into four groups according to the number of sensitized allergens: none (0-allergen group), 1 (1-allergen group), 2 (2-allergen group), and ≥3 (≥3-allergen group).
Following the patient's examination by a pediatrician, the severity of AD was assessed according to the scoring of atopic dermatitis (SCORAD) index.
Continuous variables such as age, birth weight, and eosinophil counts are expressed as means±SD. One-way ANOVA was applied to compare the differences in growth and nutritional status according to the number of sensitized food allergens, and Duncan's test was performed for post hoc analysis. The severity, eosinophil counts, total serum IgE, and time of food weaning according to the number of sensitized allergens were adjusted for age, gender, and birth weight. The z-scores were adjusted for birth weight, which was used for partial correlation analysis. All statistical analyses were performed using STATA version 11.0. A value of P<0.05 indicated statistical significance.
A total of 165 pediatric patients were enrolled in this study. They included 105 boys (63.6%) and 60 girls (36.4%). The patients ranged in age between 5 and 47 months, and the highest number of patients were <1 year old (n=77, 46.7%; data not shown). Overall, the mean birth weight was 3.29±0.40 kg, the mean eosinophil count was 694.00±784.47/µL, and the mean total serum IgE was 194.46±454.49 IU/mL. The mean SCORAD index values according to the number of sensitized food allergens were 24.91±16.02, 29.72±17.54, 26.87±15.41, and 40.37±21.14 for the 0-, 1-, 2-, and ≥3-allergen groups, respectively. As the number of sensitized food allergens increased, the SCORAD index increased (P=0.001). Total serum IgE values were 54.91±80.51, 84.01±111.06, 315.51±596.25 and 467.00±750.55 IU/mL for the 0-, 1-, 2-, and ≥3-allergen groups, respectively, and these values were significantly different (P<0.001). The time of initiating food weaning did not differ significantly among the groups.
The mean z-scores of weight for age, height for age, and weight for height were -0.06±1.03, -0.16±0.90, and 0.05±1.09, respectively. The mean z-scores of weight for age according to the number of sensitized food allergens were 0.21±1.08, 0.02±0.87, -0.14±0.96, and -0.57±1.90 for the 0-, 1-, 2-, and ≥3-allergen groups, respectively, and these scores were significantly different (P=0.006). The z-score of height for age differed significantly (P=0.018) between the 1-allergen group (1.10±0.90) and the ≥3-allergen group (-0.50±1.01). The z-score of weight for height was 0.18±1.03 in the 0-allergen group, 0.08±1.12 in the 1-allergen group, 0.12±0.93 in the 2-allergen group and 0.28±1.24 in the ≥3-allergen group (P=0.286) (Table 1).
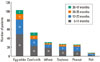
The mean number of sensitized food allergens per patient was 1.47±1.68. The most frequently sensitized food allergen was egg white, followed by cow's milk and wheat. A sensitization to egg white occurred most commonly in patients aged <1 year (n=48, 47.1%), followed by cow's milk (n=22, 42.3%) (Figure).
Following the adjustment for age, gender, and birth weight, the number of sensitized food allergens significantly correlated with the SCORAD index (r=0.308, P=0.002), time at which food weaning began (r=0.332, P=0.001), eosinophil counts (r=0.266, P=0.009), and total serum IgE (r=0.394, P<0.001) (Table 2).
Following the adjustment for birth weight, the number of sensitized food allergens significantly correlated with the z-scores of weight for age (r=-0.358, P<0.001), height for age (r=-0.278, P=0.001), and weight for height (r=-0.224, P=0.006) (Table 3).
AD is a common chronic inflammatory skin disease in children. This disease shows a significant correlation with food allergies and thus can exert a negative effect on growth and nutritional status in children who are in an active growth phase. In this study, both weight (a predictor of short-term nutritional status) and height (a predictor of relatively long-term nutritional status) significantly decreased according to the number of sensitized food allergens, and the time of initiating food weaning showed an increased delay as the number of sensitized food allergens increased. Given the negative correlations between the number of sensitized food allergens and the z-scores of weight and height for age, it is conceivable that sensitization to a food allergen can affect both growth and nutritional status in infants and young children.
It has been reported that the height for age percentile and the consumed dietary food intake are significantly less in children with two or more food allergies compared to those with a single food allergy.20 We can successfully restrict food allergens in patients by completely restricting foods that contain the offending allergens, in combination with nutritional supplementation.21 However, passive permeability of the small intestine is increased in some patients with atopic eczema,22 and continuous food restriction may increase the body's metabolic requirements.23 In particular, the requirements for energy and protein may be increased in children with AD, as energy and protein are needed for skin regeneration to compensate for the loss of skin components in AD.7 In addition, the rapid growth and high metabolic rate in infants and young children demand more nutrition per kg of body weight than that needed in adults. Previous studies have reported that growth retardation occurs during a 1-year period24 and that height is decreased more severely in children with AD than in healthy children, despite improved nutritional intake in children with AD.25 In the present study, although we could not verify a causal relationship between food restrictions and growth retardation, our results suggest a correlation between the number of sensitized food allergens and long-term/short-term nutrition and growth. Thus, adequate treatment and continuous management of growth and nutrition are crucial in infants and young children with AD.
The major sensitized food allergens are egg white, cow's milk, soybean, wheat, and peanut, all of which are important sources of protein and energy. In this study, the food allergens with the highest rates of sensitization were egg white followed by cow's milk, as in previous studies.26-29 The rates of sensitization were particularly high in infants. Low calcium intake and high risk for growth retardation have been reported in children sensitized to multiple food allergens, including cow's milk.20 Beginning at 6 months after birth, the iron reservoir continues to be depleted, while the iron requirements for rapid growth are increased at this age.30 Thus, in children who have restricted intake of cow's milk and egg white, an appropriate supply of alternative foods should be provided to meet the recommended requirements for protein and calcium.7
In the present study, the number of sensitized food allergens significantly correlated with the number of eosinophils, total IgE levels, and the time at which food weaning was initiated, as previously reported.29,31,32 Food weaning is typically initiated at the age of 4 to 6 months, depending on the development status of the infant.33 Although it has been recommended that AD-prone infants begin weaning after the age of 6 months, there is still no definitive evidence that this prevents the occurrence of AD.34 In our study, we observed that the initiation of food weaning was increasingly delayed as the number of sensitized food allergens increased. Based on reports that the prevalence of food allergies is higher at younger ages, many parents of children with AD regard food allergy as the main cause of the AD, and hence hesitate to begin weaning or eliminating foods containing major food allergens without a definitive diagnosis. When allergic tests in a child are positive for egg white or cow's milk, the parents tend to restrict this food without any supplementation, resulting in an inadequate nutritional intake.23 However, up to the age of 1 year, food-related problems can occur in children in the absence of food allergies.35 Thus, we should avoid unnecessary food restrictions by keeping in mind that not all food-related symptoms occurring during the food weaning period are attributable to food allergy. In all infants with AD, it is necessary to begin weaning foods at an appropriate time while simultaneously providing foods to satisfy growth patterns and nutritional requirements.
In this study, we could not evaluate growth patterns according to nutritional intake, and any specific food restrictions of the subjects were not verified. Nevertheless, we confirmed that an increased number of sensitized food allergens is associated with negative effects on both short-term and long-term nutritional status and may cause growth retardation. Further studies are required to determine the relationship between nutritional intake, growth, and nutritional status.
In conclusion, the results of this study suggest that food allergies may be a risk factor for growth retardation in infants and young children with AD, and the risk increases with an increase in the number of sensitized food allergens. The meticulous and continuous evaluation and management of both growth and nutritional status should be considered in AD patients with a high number of sensitized food allergens.
Figures and Tables
References
1. Kim KE. Genetics of atopy and asthma. Pediatr Allergy Respir Dis. 1999. 9:343–350.
2. Elliott K, Forrest S. Bieber T, Leung DYM, editors. Genetics of atopic dermatitis. Atopic dermatitis. 2002. New York: Marcel Dekker;81–110.
3. Wang J, Sampson HA. Nutrition in infant allergy: a step in the right direction. Holist Nurs Pract. 2006. 20:299–302.
4. Arshad SH. Food allergen avoidance in primary prevention of food allergy. Allergy. 2001. 56:Suppl 67. 113–116.
5. Sicherer SH, Sampson HA. 9. Food allergy. J Allergy Clin Immunol. 2006. 117:S470–S475.
6. Sicherer SH, Sampson HA. Food hypersensitivity and atopic dermatitis: pathophysiology, epidemiology, diagnosis, and management. J Allergy Clin Immunol. 1999. 104:S114–S122.
7. Mofidi S. Nutritional management of pediatric food hypersensitivity. Pediatrics. 2003. 111:1645–1653.
8. Fox AT, Du Toit G, Lang A, Lack G. Food allergy as a risk factor for nutritional rickets. Pediatr Allergy Immunol. 2004. 15:566–569.
9. Lee DG, Rho YI, Moon KR. Assessment of nutritional status in hospitalized pediatric patients. Korean J Pediatr Gastroenterol Nutr. 2001. 4:83–91.
10. Blackburn GL, Benotti PN, Bistrian BR, Bothe A, Maini BS, Schlamm HT, Smith MF. Nutritional assessment and treatment of hospital malnutrition. Infusionsther Klin Ernahr. 1979. 6:238–250.
11. Kristmundsdottir F, David TJ. Growth impairment in children with atopic eczema. J R Soc Med. 1987. 80:9–12.
12. Massarano AA, Hollis S, Devlin J, David TJ. Growth in atopic eczema. Arch Dis Child. 1993. 68:677–679.
13. Katz KA, Mahlberg MJ, Honig PJ, Yan AC. Rice nightmare: kwashiorkor in 2 Philadelphia-area infants fed rice dream beverage. J Am Acad Dermatol. 2005. 52:S69–S72.
14. Noimark L, Cox HE. Nutritional problems related to food allergy in childhood. Pediatr Allergy Immunol. 2008. 19:188–195.
15. Chung SJ, Han YS, Chung SW, Ahn KM, Park HY, Lee SI, Cho YY, Choi HM. Marasmus and kwashiorkor by nutritional ignorance related to vegetarian diet and infants with atopic dermatitis in South Korea. Korean J Nutr. 2004. 37:540–549.
16. Groetch M. Metcalfe DD, Sampson HA, Simon RA, editors. Diet and nutrition. Food allergy: adverse reactions to foods and food additives. 2008. 4th ed. Malden: Blackwell;482–497.
17. Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venereol Suppl (Stockh). 1980. 92:44–47.
18. Dibley MJ, Staehling N, Nieburg P, Trowbridge FL. Interpretation of Z-score anthropometric indicators derived from the international growth reference. Am J Clin Nutr. 1987. 46:749–762.
19. de Onis M, Blossner M. WHO. WHO Global database on child growth and malnutrition. 1997. Geneva: WHO.
20. Christie L, Hine RJ, Parker JG, Burks W. Food allergies in children affect nutrient intake and growth. J Am Diet Assoc. 2002. 102:1648–1651.
21. Grimshaw KE. Dietary management of food allergy in children. Proc Nutr Soc. 2006. 65:412–417.
22. Ukabam SO, Mann RJ, Cooper BT. Small intestinal permeability to sugars in patients with atopic eczema. Br J Dermatol. 1984. 110:649–652.
23. Eggesbø M, Botten G, Stigum H. Restricted diets in children with reactions to milk and egg perceived by their parents. J Pediatr. 2001. 139:583–587.
24. Agostoni C, Grandi F, Scaglioni S, Gianni ML, Torcoletti M, Radaelli G, Fiocchi A, Riva E. Growth pattern of breastfed and nonbreastfed infants with atopic dermatitis in the first year of life. Pediatrics. 2000. 106:E73.
25. Kim JH, Lee HC, Jang JH, Ahn KM, Han YS, Lee SI. Risk factors influencing growth in children with atopic dermatitis. Pediatr Allergy Respir Dis. 2008. 18:339–348.
26. Sampson HA. Update on food allergy. J Allergy Clin Immunol. 2004. 113:805–819.
27. Han YS, Chung SJ, Cho YY, Choi HM, Ahn KM, Lee SI. Analysis of the rate of sensitization to food allergen in children with atopic dermatitis. Korean J Community Nutr. 2004. 9:90–97.
28. Cho GR, Kim MJ, Kim JE, Jung JA. A comparison of the sensitization rate to the cow's milk, egg white and soybean in atopic dermatitis at a single institution in 2002 and 2007. Pediatr Allergy Respir Dis. 2008. 18:283–291.
29. Na HY, Song YH, Kim BJ, Yu JH, Hong SJ, Lee SY. Allergen sensitization of severe atopic dermatitis in children under 2 years. Pediatr Allergy Respir Dis. 2009. 19:146–154.
30. Gartner LM, Morton J, Lawrence RA, Naylor AJ, O'Hare D, Schanler RJ, Eidelman AI. Breastfeeding and the use of human milk. Pediatrics. 2005. 115:496–506.
31. Sampson HA. Role of immediate hypersensitivity in the pathogenesis of atopic dermatitis. Allergy. 1989. 44:Suppl 9. 52–58.
32. Weller PF. Eosinophilia. J Allergy Clin Immunol. 1984. 73:1–14.
33. Butte N, Cobb K, Dwyer J, Graney L, Heird W, Rickard K. The start healthy feeding guidelines for infants and toddlers. J Am Diet Assoc. 2004. 104:442–454.
34. Greer FR, Sicherer SH, Burks AW. Effects of early nutritional interventions on the development of atopic disease in infants and children: the role of maternal dietary restriction, breastfeeding, timing of introduction of complementary foods, and hydrolyzed formulas. Pediatrics. 2008. 121:183–191.
35. Luccioli S, Ross M, Labiner-Wolfe J, Fein SB. Maternally reported food allergies and other food-related health problems in infants: characteristics and associated factors. Pediatrics. 2008. 122:Suppl 2. S105–S112.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download