Abstract
Purpose
The aim of this study was to investigate bronchodilator responsiveness (BDR) following methacholine-induced bronchoconstriction and to determine differences in BDR according to clinical parameters in children with asthma.
Methods
The methacholine challenge test was performed in 145 children with mild to moderate asthma, and the provocative concentration causing a 20% decline in FEV1 (PC20) was determined. Immediately after the challenge test, patients were asked to inhale short-acting β2-agonists (SABAs) to achieve BDR, which was assessed as the change in FEV1% predicted×100/post-methacholine FEV1% predicted. For each subject, the asthma medication, blood eosinophil count, serum total IgE, serum eosinophil cationic protein level, and skin prick test result were assessed.
Results
The FEV1 (mean±SD) values of the 145 patients were 90.5±10.9% predicted, 64.2±11.5% predicted, and 86.2±11.2% predicted before and after methacholine inhalation, and following the administration of a SABA, respectively. The BDR did not differ significantly according to asthma medication, age, or gender. However, BDR in the atopy group (37.4±17.7%) was significantly higher than that in the non-atopy group (30.5±10.7%; P=0.037). Patients with blood eosinophilia (38.6±18.1%) displayed increased BDR compared with patients without eosinophilia (32.0±13.8%; P=0.037).
The reversibility of bronchoconstriction with β2-adrenergic agonists and increased airway responsiveness to a variety of stimuli are hallmarks of asthma.1 Current treatment guidelines recommend the use of inhaled corticosteroids and β2-adrenergic agonists for individuals with mild to moderate persistent asthma.2 However, acute asthma attacks caused by extensive airway obstruction following airway smooth muscle constriction sometimes occur in patients receiving asthma medications.3 Inhaled short-acting β2-adrenergic agonists (SABAs) play an important role in the treatment of acute bronchoconstriction.4
Bronchodilator responsiveness (BDR) is the improvement of forced expiratory volume in 1 second (FEV1) after inhalation of β2-agonists and is typically measured as the change in airflow after the administration of SABAs. BDR is commonly used to assess the severity of asthma and to guide pharmacological treatment. However, the responses to inhaled β2-adrenergic agonists can be highly variable.5
Airway hyper-responsiveness (AHR), defined as an exaggerated bronchoconstrictive response of the airways to the numerous stimuli that provoke bronchial obstruction and inflammation, is a characteristic feature of asthma.6-8 The evaluation of AHR is useful for the diagnosis of asthma, the determination of asthma severity, and the therapeutic responses to bronchodilators. Methacholine inhalation elicits acute bronchial obstruction in individuals with asthma, in part by direct cholinergic stimulation. Thus, the reversal of methacholine-induced bronchoconstriction by bronchodilators is a useful and standardized model for the determination of airway responsiveness to bronchodilators.9,10
The knowledge of factors affecting BDR in children with asthma is limited. Understanding the implications of a response to a bronchodilator may help clinicians to more effectively assess asthma treatment regimens in the outpatient setting. The aim of this study was to determine the response to bronchodilators after methacholine-induced bronchoconstriction and to investigate the relationship between BDR and clinical parameters in children with mild to moderate asthma.
Children, aged 6-18 years, who had physician-diagnosed mild to moderate asthma and a history of episodic wheezing and/or dyspnea that resolved with bronchodilators during the previous year were enrolled in this study (n=145). The patients were followed at the allergy clinic of the Anam Hospital of Korea University. The clinical severity of asthma was assessed according to the National Asthma Education and Prevention Program.11 Treatment included inhaled SABAs on demand for the relief of symptoms, with or without control medications; inhaled corticosteroids; leukotriene receptor antagonists; or inhaled long-acting β2-agonists (LABAs). Patients with a history of near-fatal asthma, major exacerbations necessitating the use of systemic corticosteroids, or other respiratory diseases were excluded from the study.
Parents provided written informed consent for their children to participate in the study. The study protocol was approved by the Institutional Review Board of Anam Hospital.
Spirometry (FEV1 and forced vital capacity [FVC]) was performed using a computerized spirometer (Microspiro-HI 298; Chest; Tokyo, Japan), in accordance with the recommendations of the American Thoracic Society.12 All patients were required to achieve a FEV1 of at least 70% of the predicted value.
Following the establishment of a baseline spirometry value, all patients underwent bronchoprovocation with increasing concentrations of methacholine. Methacholine inhalation tests were performed using a modification of the method described by Chai et al.13 At the time of the test, all patients had been free of acute respiratory tract infection and asthma exacerbation for 4 weeks. All patients were asked to discontinue the use of any inhaled SABA for 24 hours, and any inhaled LABA or corticosteroid for 7 days prior to the test. Methacholine (Sigma Diagnostics, St. Louis, MO, USA) solutions were prepared at concentrations of 0.075, 0.15, 0.3, 0.625, 1.25, 2.5, 5, 10, and 25 mg/mL in buffered saline solution (pH 7.4). A Rosenthal-French dosimeter (Laboratory for Applied Immunology, Baltimore, MD, USA), triggered by a solenoid valve set to remain open for 0.6 second, was used to administer an aerosol from a DeVilbiss 646 nebulizer (DeVilbiss Health Care, Somerset, PA, USA) with pressurized air at 20 psi. Each patient performed inhalations of five inspiratory capacity breaths of buffered saline solution containing increasing concentrations of methacholine, at 5-minute intervals. This gave an output of 0.009±0.0014 mL (mean±SD) per inhalation. The FEV1 and FVC were measured 90 seconds after inhalation of each concentration, and the largest of triplicate FEV1 or FVC values was used for the analysis. The procedure was terminated when the FEV1 decreased by more than 20% of the post-saline value or when the highest methacholine concentration (25 mg/mL) was reached. The percentage decline of the FEV1 from the post-saline value was plotted against the log concentration of inhaled methacholine. The provocative concentration (PC20) and provocative cumulative dose of methacholine producing a 20% fall in FEV1 (PD20) were calculated by interpolating between two adjacent data points. The total cumulative dose (c.u.), one dose unit being one inhalation of 1 mg/mL of methacholine, was calculated. All patients displayed a PC20 of less than 16 mg/mL.
Immediately after the cessation of methacholine upon a fall in FEV1 to more than 20% of the post-saline value, a bronchodilator responsiveness test to inhaled salbutamol (Ventolin Evohaler; GlaxoSmithKline, London, UK) was performed. Four separate doses of salbutamol (400 µg) were administered from a metered dose inhaler via a spacer (AeroChamber Plus; Trudell Medical International, Ontario, Canada); spirometry was performed after 15 minutes (post-BD FEV1 and post-BD FVC). The BDR was defined as the change in FEV1% predicted×100/post-methacholine FEV1% predicted.14
The skin prick test was performed using 13 common aeroallergens, and atopy was defined as the presence of at least one positive reaction (mean wheal diameter of >3 mm) to these allergens.
The number of peripheral blood eosinophils was counted from blood samples containing EDTA, using an automated hematology analyzer (Coulter Counter STKS; Beckman Coulter, Fullerton, CA, USA). The serum total IgE level was measured using a Coat-A-Count Total IgE IRMA (Diagnostic Products Co., Los Angeles, CA, USA) according to the manufacturer's instructions. The serum eosinophil cationic protein (ECP) level was measured using a commercially available fluoroimmunoassay kit (Pharmacia ECP UniCAP System FEIA; Pharmacia Diagnostics, Uppsala, Sweden) with a detection limit of less than 2.0 µg/L.
Both FEV1 and FVC were expressed as a percentage of the predicted value based on data from our local population. The values for PC20, PD20, blood eosinophil counts, serum total IgE, and serum ECP were log transformed prior to statistical analysis. Data are presented as means±SD. Variables were compared between two groups using Student's t-test or a chi-squared test, as appropriate. One-way ANOVA and a post hoc test were performed to account for asthma medication-related differences in baseline FEV1, the fall in FEV1 during methacholine challenge (ΔFEV1), post-BD FEV1, and BDR. All statistical analyses were performed using SPSS software (SPSS Inc., Chicago, IL, USA). Values of P<0.05 were considered to indicate statistical significance.
A total of 145 children with asthma were enrolled in this study. The clinical characteristics of the subjects are shown in Table 1. Among the 145 patients, 23 used an inhaled SABA, 29 received a leukotriene receptor antagonist, 32 received an inhaled corticosteroid, and 61 received the combination of an inhaled corticosteroid plus a LABA to relieve asthma symptoms. The mean (±SD) age of the patients was 9.4 (±2.7) years.
Table 2 shows the pulmonary function parameters of the patients. The FEV1 values (mean±SD) were 90.5±10.9% predicted, 64.2±11.5% predicted, and 86.2±11.2% predicted before and after methacholine inhalation, and after SABA administration, respectively.
The bronchodilator responsiveness and methacholine inhaled dose are shown in Table 3. The mean (±SD) percentage reduction of FEV1 after methacholine inhalation was 29.2±8.1%, and the mean (±SD) BDR was 36.7±17.0%. A total of 109 patients (75.2%) recovered to >90% of the pre-challenge FEV1 value. The geometric means (range of 1 SD) of the methacholine PC20 and total methacholine inhaled dose were 2.60 (range, 0.66-10.3) mg/mL and 33.9 (range, 8.13-141.3) c.u., respectively.
The FEV1% predicted values before and after methacholine challenge and after bronchodilator use as well as the BDR did not differ significantly according to the asthma medication used (Fig. 1).
The pre- and post-methacholine and post-BD FEV1 did not differ between the atopic and non-atopic patients, although the BDR was significantly higher in atopic subjects compared with non-atopic subjects (37.4±17.7% vs. 30.5±10.7%, respectively; P=0.037; Fig. 2). Children who displayed peripheral blood eosinophil counts of ≥4% showed significantly higher BDR than those with eosinophil counts of <4% (38.6±18.1% vs. 32.0±13.8%, respectively; P=0.037; Fig. 3).
The assessment of responsiveness to bronchodilators is essential for the diagnosis and treatment of asthma. Current treatment guidelines recommend the use of an inhaled SABA to relieve symptoms in individuals with mild to moderate asthma.2 In the present study, the BDR and the clinical factors that may affect BDR were evaluated in children with mild to moderate asthma. Asthmatic patients with atopy and/or eosinophilia displayed an increased responsiveness to bronchodilators after methacholine-induced bronchoconstriction.
BDR is typically assessed as the change in airflow before versus after the administration of a β2-agonist; however, the baseline airway tone may influence BDR.14 It is expected that airflow would not increase if the airways at baseline were fully dilated by asthma treatment. The present study addressed this issue by measuring BDR following methacholine-induced bronchoconstriction. This challenge-rescue method of bronchodilation measurement was suggested in the 1970s as a method for overcoming the variability in FEV1 prior to measuring bronchodilation.15 In addition, chemical challenges such as the methacholine test have the advantages of being easily controlled, using standard doses, and producing immediate bronchoconstriction.16 However, as these situations may differ from the actual clinical setting, it is reasonable to question the relevance of this artificial model of bronchoconstriction and bronchodilation to a real asthma attack. Several studies9,10,17,18 have demonstrated that methacholine-induced bronchoconstriction and its reversal by inhaled bronchodilators is a useful and standardized model for estimating the effectiveness of bronchodilators.
In this study, BDR was defined as the change in FEV1% predicted over the pre-bronchodilator value, which has been suggested to be the best of several indices of bronchodilator response because it accounts for the confounding effects of height, gender, and pre-bronchodilator FEV1.14,19-21 The mean BDR in this study was 36.7%, which is comparable to previously reported BDR values of >30% after methacholine-induced bronchoconstriction in asthmatic patients.9,22
For the determination of BDR in the present study, the patients were instructed to inhale 400 µg of salbutamol. Although 800 µg of salbutamol have been administered to obtain near maximal bronchodilation,23 the generally recommended dose for BDR determination is 400 µg.24 However, residual airflow limitations may exist, which could explain our finding that the mean post-BD FEV1 was slightly lower than the mean pre-challenge baseline FEV1. This may occur because of variable geometric factors, including hypertrophy or hyperplasia of the mucous glands and smooth muscle, interstitial edema, and thickening of the reticular lamina, which are not acutely influenced by smooth muscle relaxants.19
An interesting finding of this study is the higher BDR observed in the atopic group compared with the non-atopic group. The reason for this difference is not clear. Previous studies have not reported a correlation between BDR and atopy among children from families with asthma or the general population,25 while other studies have described a significantly higher BDR in adults with atopic asthma than in those with non-atopic asthma.26 In one study, asthmatic children with atopy displayed a higher BDR than those without atopy, but the difference was not statistically significant.27 These discrepancies may be attributable to differences among the doses of inhaled SABAs or patient selection. In previous studies, 200 µg of a SABA were used in patients with stable asthma who did not display reduced FEV1. Although it has been suggested that atopy and BDR have independent patterns of inheritance, the presence of atopy may correlate with the increased airway responsiveness that follows bronchodilator inhalation.
The present finding of higher BDR in patients with high blood eosinophil counts is consistent with previous observations. Silvestri et al.27 reported a significant positive relationship between salbutamol-induced changes in FEV1% and blood eosinophilia in 92 children with asthma. A study of serial bronchial biopsy specimens from nine patients with asthma demonstrated a significant correlation between changes in BDR and changes in eosinophil counts after 8 weeks of inhaled corticosteroid therapy.28 Furthermore, in adult asthmatics, the response to a β2-agonist was closely related to the absolute number of eosinophilis in the peripheral blood.29 These results suggest that an increased number of eosinophils is not a direct marker of reversibility and that BDR may be a marker for inflammation.30
Another point of discussion is the dose of methacholine administered. As subjects with a higher methacholine reactivity received a lower dose of methacholine to achieve the same level of bronchoconstriction, it is possible that the higher BDR in these patients was a reflection of the lower levels of bronchoconstrictive agent received.9 It can be argued that the differences in BDR observed in this study are due to the different doses of methacholine inhaled. However, this is unlikely because the doses of methacholine did not differ among the patient subgroups. In contrast, subjects with eosinophilia or atopy displayed lower PC20 (data not shown). A higher BDR in these groups was more likely to be directly associated with a lower PC20, rather than eosinophila. Nevertheless, it is clear that patients with eosinophilia or atopy exhibited a much greater response to bronchodilators after induced bronchoconstriction.
BDR did not differ according to the asthma medication. In this study, immediately after methacholine challenge, the FEV1 decreased by 30%, corresponding to a moderate to severe asthma attack, with no apparent differences among the medication subgroups. A larger reduction in lung function can occur with acute severe asthma attacks, and the loss of responsiveness to bronchodilators most likely corresponds to a greater degree.18,30 Although a failure to respond to bronchodilators may also be associated with other factors such as airway mucus plugging and mucosal edema,31 the results of this study indicate reduced BDR with acute asthma exacerbation in non-atopic and/or non-eosinophilic patients.
Low BDR indicates a persistent airflow limitation during an acute asthma attack. The pre-challenge baseline FEV1 in non-atopic or non-eosinophilic patients did not differ from that in the atopic or eosinophilia group. In addition, a low post-BD FEV1/FVC, an index of airway remodeling in patients with asthma, was not observed in these groups, meaning that fixed airway obstruction can be excluded.32 At present, the guidelines recommend a SABA for treatment of episodic bronchoconstriction. We believe it is important that clinicians are aware of the asthma patient parameters related to effective bronchodilator responsiveness. This information will be helpful in current patient treatment regimens.
In conclusion, the results of this study show that the response to a bronchodilator after methacholine-induced bronchoconstriction differed between atopic and non-atopic asthmatic patients and varied according to the presence of blood eosinophilia. These results raise the possibility of a reduced bronchodilator response in the clinical setting of a severe asthma attack, particularly in patients without atopy and/or eosinophilia.
Figures and Tables
Fig. 1
FEV1% predicted before methacholine, after methacholine (last concentration), and 15 min after bronchodilator administration, and bronchodilator responsiveness (BDR) according to asthma medication.  , inhaled short-acting β2-agonists on demand;
, inhaled short-acting β2-agonists on demand;  , leukotriene receptor antagonists;
, leukotriene receptor antagonists;  , inhaled corticosteroids alone;
, inhaled corticosteroids alone;  inhaled long-acting β2-agonists and corticosteroid combination.
inhaled long-acting β2-agonists and corticosteroid combination.
 , inhaled short-acting β2-agonists on demand;
, inhaled short-acting β2-agonists on demand;  , leukotriene receptor antagonists;
, leukotriene receptor antagonists;  , inhaled corticosteroids alone;
, inhaled corticosteroids alone;  inhaled long-acting β2-agonists and corticosteroid combination.
inhaled long-acting β2-agonists and corticosteroid combination.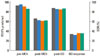
Fig. 2
FEV1% predicted before methacholine, after methacholine (last concentration), and 15 min after bronchodilator administration, and bronchodilator responsiveness (BDR) according to the presence ( ) or absence (
) or absence ( ) of atopy.
) of atopy.
 ) or absence (
) or absence ( ) of atopy.
) of atopy.
Fig. 3
FEV1% predicted before methacholine, after methacholine (last concentration), and 15 min after bronchodilator administration, and bronchodilator responsiveness (BDR) based on blood eosinophil count.  , ≥ 4%;
, ≥ 4%;  , < 4%.
, < 4%.
 , ≥ 4%;
, ≥ 4%;  , < 4%.
, < 4%.
Table 2
Post-methacholine and post-bronchodilator pulmonary function parameters of the children with asthma

Table 3
Bronchodilator responsiveness and inhaled methacholine dose of the children with asthma

*Mean±SD; †Subjects recovering to ≥90% of baseline FEV1; ‡Geometric mean (range of 1 SD).
FEV1, forced expiratory volume in 1 sec; BDR, bronchodilator responsiveness; PC20; provocative concentration of methacholine causing a 20% decline in FEV1; PD20, provocative cumulative dose of methacholine causing a 20% decline in FEV1.
ACKNOWLEDGMENTS
This study was supported by a research grant from the Environmental Research Center Project (2010), Ministry of Environment, Republic of Korea.
References
1. Masoli M, Fabian D, Holt S, Beasley R. The global burden of asthma: executive summary of the GINA Dissemination Committee report. Allergy. 2004. 59:469–478.
2. Bousquet J, Clark TJ, Hurd S, Khaltaev N, Lenfant C, O'Byrne P, Sheffer A. GINA guidelines on asthma and beyond. Allergy. 2007. 62:102–112.
3. Malakauskas K, Sitkauskiene B, Stravinskaite K, Sakalauskas R. Dyspnea perception and reversibility of methacholine-induced unlimited airway narrowing in asthmatics. J Asthma. 2006. 43:463–467.
4. Bateman ED, Hurd SS, Barnes PJ, Bousquet J, Drazen JM, FitzGerald M, Gibson P, Ohta K, O'Byrne P, Pedersen SE, Pizzichini E, Sullivan SD, Wenzel SE, Zar HJ. Global strategy for asthma management and prevention: GINA executive summary. Eur Respir J. 2008. 31:143–178.
5. Abramson MJ, Walters J, Walters EH. Adverse effects of beta-agonists: are they clinically relevant? Am J Respir Med. 2003. 2:287–297.
6. Juniper EF, Frith PA, Hargreave FE. Airway responsiveness to histamine and methacholine: relationship to minimum treatment to control symptoms of asthma. Thorax. 1981. 36:575–579.
7. Fourie PR, Joubert JR. Determination of airway hyper-reactivity in asthmatic children: a comparison among exercise, nebulized water, and histamine challenge. Pediatr Pulmonol. 1988. 4:2–7.
8. Smith L, McFadden ER Jr. Bronchial hyperreactivity revisited. Ann Allergy Asthma Immunol. 1995. 74:454–469.
9. Parker AL. Airway reactivity is a determinant of bronchodilator responsiveness after methacholine-induced bronchoconstriction. J Asthma. 2004. 41:671–677.
10. Haney S, Hancox RJ. Recovery from bronchoconstriction and bronchodilator tolerance. Clin Rev Allergy Immunol. 2006. 31:181–196.
11. National Asthma Education and Prevention Program. Guidelines for the diagnosis and management of asthma: expert panel report 2. 1997. Bethesda: US Dept of Health and Human Services;(NIH publication; no. 97-4051).
12. American Thoracic Society. Standardization of spirometry, 1994 update. Am J Respir Crit Care Med. 1995. 152:1107–1136.
13. Chai H, Farr RS, Froehlich LA, Mathison DA, McLean JA, Rosenthal RR, Sheffer AL, Spector SL, Townley RG. Standardization of bronchial inhalation challenge procedures. J Allergy Clin Immunol. 1975. 56:323–327.
14. Dales RE, Spitzer WO, Tousignant P, Schechter M, Suissa S. Clinical interpretation of airway response to a bronchodilator. Epidemiologic considerations. Am Rev Respir Dis. 1988. 138:317–320.
15. Smith AP, Orehek J, Charpin J. Bronchodilator drug tests in normal subjects [proceedings]. Br J Dis Chest. 1977. 71:234–236.
16. Crapo RO, Casaburi R, Coates AL, Enright PL, Hankinson JL, Irvin CG, MacIntyre NR, McKay RT, Wanger JS, Anderson SD, Cockcroft DW, Fish JE, Sterk PJ. Guidelines for methacholine and exercise challenge testing-1999. Am J Respir Crit Care Med. 2000. 161:309–329.
17. Parker AL. Aging does not affect beta-agonist responsiveness after methacholine-induced bronchoconstriction. J Am Geriatr Soc. 2004. 52:388–392.
18. van der Woude HJ, Winter TH, Aalbers R. Decreased bronchodilating effect of salbutamol in relieving methacholine induced moderate to severe bronchoconstriction during high dose treatment with long acting β2 agonists. Thorax. 2001. 56:529–535.
19. Waalkens HJ, Merkus PJ, van Essen-Zandvliet EE, Brand PL, Gerritsen J, Duiverman EJ, Kerrebijn KF, Knol KK, Quanjer PH. Dutch CNSLD Study Group. Assessment of bronchodilator response in children with asthma. Eur Respir J. 1993. 6:645–651.
20. Brand PL, Quanjer PH, Postma DS, Kerstjens HA, Koeter GH, Dekhuijzen PN, Sluiter HJ. Dutch Chronic Non-Specific Lung Disease (CNSLD) Study Group. Interpretation of bronchodilator response in patients with obstructive airways disease. Thorax. 1992. 47:429–436.
21. Dundas I, Chan EY, Bridge PD, McKenzie SA. Diagnostic accuracy of bronchodilator responsiveness in wheezy children. Thorax. 2005. 60:13–16.
22. Hancox RJ, Aldridge RE, Cowan JO, Flannery EM, Herbison GP, McLachlan CR, Town GI, Taylor DR. Tolerance to beta-agonists during acute bronchoconstriction. Eur Respir J. 1999. 14:283–287.
23. Merkus PJ, Rooda HM, van Essen-Zandvliet EE, Duiverman EJ, Quanjer PH, Kerrebijn KF. Assessment of bronchodilatation after spontaneous recovery from a histamine challenge in asthmatic children. Thorax. 1992. 47:355–359.
24. Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, Coates A, van der, Gustafsson P, Hankinson J, Jensen R, Johnson DC, MacIntyre N, McKay R, Miller MR, Navajas D, Pedersen OF, Wanger J. Interpretative strategies for lung function tests. Eur Respir J. 2005. 26:948–968.
25. Kumar R, Wang B, Wang X, Chen C, Yang J, Fu L, Xu X. Bronchodilator responses in Chinese children from asthma index families and the general population. J Allergy Clin Immunol. 2006. 117:1257–1263.
26. Zietkowski Z, Bodzenta-Lukaszyk A, Tomasiak MM, Skiepko R, Szmitkowski M. Comparison of exhaled nitric oxide measurement with conventional tests in steroid-naïve asthma patients. J Investig Allergol Clin Immunol. 2006. 16:239–246.
27. Silvestri M, Sabatini F, Sale R, Defilippi AC, Fregonese L, Battistini E, Biraghi MG, Rossi GA. Correlations between exhaled nitric oxide levels, blood eosinophilia, and airway obstruction reversibility in childhood asthma are detectable only in atopic individuals. Pediatr Pulmonol. 2003. 35:358–363.
28. Faul JL, Demers EA, Burke CM, Poulter LW. Alterations in airway inflammation and lung function during corticosteroid therapy for atopic asthma. Chest. 2002. 121:1414–1420.
29. Sitkauskiene B, Sakalauskas R, Malakauskas K, Lötvall J. Reversibility to a β2-agonist in COPD: relationship to atopy and neutrophil activation. Respir Med. 2003. 97:591–598.
30. Tantisira KG, Fuhlbrigge AL, Tonascia J, Van Natta M, Zeiger RS, Strunk RC, Szefler SJ, Weiss ST; Childhood Asthma Management Program Research Group. Bronchodilation and bronchoconstriction: predictors of future lung function in childhood asthma. J Allergy Clin Immunol. 2006. 117:1264–1271.
31. Djukanović R, Roche WR, Wilson JW, Beasley CR, Twentyman OP, Howarth RH, Holgate ST. Mucosal inflammation in asthma. Am Rev Respir Dis. 1990. 142:434–457.
32. Rasmussen F, Taylor DR, Flannery EM, Cowan JO, Greene JM, Herbison GP, Sears MR. Risk factors for airway remodeling in asthma manifested by a low postbronchodilator FEV1/vital capacity ratio: a longitudinal population study from childhood to adulthood. Am J Respir Crit Care Med. 2002. 165:1480–1488.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download