Abstract
Purpose
Severe asthma is characterized by high medication requirements to maintain good disease control or by persistent symptoms despite high medication use. The transfer of bone marrow-derived mesenchymal stem cells (BMDMSCs) to the injured lungs is a possible treatment for severe asthma. This study investigated the therapeutic effects of BMDMSCs in airway remodeling and inflammation in an experimental toluene diisocyanate (TDI)-induced asthma animal model of severe asthma.
Methods
BMDMSCs were transferred into rats after TDI inhalation. Bronchoalveolar lavage (BAL) cell profiles, histological changes including an inflammatory index and goblet cell hyperplasia, and the airway response to methacholine using plethysmography were analyzed. Smooth muscle actin (SMA) and proliferating cell nuclear antigen (PCNA) protein expression were observed in lung tissue using immunohistochemical staining. The collagen content was measured in lung tissue sections and lung extracts using Masson's trichrome staining and an immunoassay kit.
Results
The numbers of inflammatory cells in BAL fluid, histological inflammatory index, airway response to methacholine, number of goblet cells, and amount of collagen were increased in TDI-treated rats compared with sham rats (P=0.05-0.002). BMDMSC transfer significantly reduced the TDI-induced increase in the inflammatory index and numbers of eosinophils and neutrophils in BAL fluid to levels seen in sham-treated rats (P<0.05). BMDMSC transfer significantly reduced the number of goblet cells, collagen deposition, and immune staining for SMA and PCNA with concomitant normalization of the airway response to methacholine.
Asthma is a common heterogeneous respiratory disease characterized by obstruction and symptoms that are caused by airway inflammation and remodeling.1 Inflammation and remodeling may contribute to airway hyperresponsiveness (AHR) and chronic airway obstruction.2 Current therapies targeting airway inflammation relieve and prevent symptoms in the majority of patients, although some patients experience persistent symptoms and a progressive decline in lung function, leading to the development of fixed airway obstruction and excessive airway narrowing.3,4 Patients with difficult-to-control asthma commonly take doses of inhaled therapy higher than standard doses and/or oral corticosteroid therapy.3,5 From a practical standpoint, patients whose asthma is not controlled by high-dose inhaled corticosteroid therapy are considered to have difficult-to-control/therapy-resistant asthma.3,5 Although a very small proportion of the patients with asthma have difficult-to-control/therapy-resistant asthma, these patients consume disproportional amounts of medical resources in terms of both time and money.6 What underlies this decline in lung function is unclear, but it may reflect chronic progressive airway wall remodeling. The pathological features of airway remodeling include goblet cell and mucosal gland hyperplasia, the deposition of extracellular matrix in the submucosa, smooth muscle cell hypertrophy/hyperplasia, and hyperplasia of fibroblasts/myofibroblasts.3,5 The increase in airway wall thickness, collagen content, and airway smooth muscle mass have been associated, although inconsistently, with asthma severity.7-9 The possibility that remodeling can contribute significantly to fixed airway obstruction is supported by the persistence of the latter in some patients with severe asthma under maximal treatment that eliminates airway inflammation.10 The physiological consequences of these changes remain uncertain, partially because these changes are not fully reversed by current asthma therapy.11,12 Therefore, developing a strategy to reverse airway remodeling is important so as to prevent the progressive decline of lung function in patients with asthma that persists despite long-term conventional treatments.
In recent years, interest the stromal cell system has increased; this includes marrow-derived stromal cells that support hematopoiesis, mesenchymal stem cells and their progeny, and connective tissue cells, such as osteocytes, chondrocytes, tenocytes, adipocytes, and smooth muscle cells.13 Bone marrow-derived mesenchymal stem cells (BMDMSCs) are a group of plastic adherent CD45-negative CD44H-positive cells that are capable of differentiating into a variety of cell types depending on culture conditions, including endothelial, epithelial, and neuronal cells, and adipocytes.13 When BMDMSCs are infused into mice, they can be found in the liver, muscles, heart, intestines, and lungs, with phenotypic characteristics of the cells in the organ where they reside.14 In the lungs, BMDMSCs have been detected as types I and II alveolar epithelial cells, endothelial cells, fibroblasts, and bronchial epithelial cells,15 which raises the exciting possibility that BMDMSCs may have therapeutic potential.
After venous injection, BMDMSCs must pass through the lungs. Previous studies suggest higher engraftment of BMDMSCs into the lungs compared with other organs.16,17 Previously, we reported that BMDMSCs engrafted at low levels when administered to mice challenged with bleomycin, but significantly reduced the extent of inflammation and fibrosis in the lung,13 as Ortiz et al.18 demonstrated. This suggested a therapeutic strategy using BMDMSCs in patients with severe/difficult-to-control asthma. To our knowledge, however, no trial has examined BMDMSC transfer in experimental asthma. Therefore, this study assessed the effects of BMDMSCs on airway inflammation and remodeling in a toluene diisocyanate (TDI)-induced asthma mouse model. TDI-induced asthma results in chronic airways inflammation, which increases epithelial cell damage and airway remodeling.19
First, BMDMSCs were isolated from the bone marrow of Sprague-Dawley (SD) rats (Charles River Technology, Waltham, MA, USA) without contaminating hematopoietic cells, as described previously.20 Briefly, bone marrow cells were collected by flushing the femurs, tibias, and iliac crests of 6-week-old rats with phosphate-buffered saline (PBS) supplemented with 2% fetal bovine serum (FBS; GIBCO, Paisley, UK). The collected cells were cultured and maintained in T75 flasks containing Dulbecco's modified Eagle's medium and 10% FBS, supplemented with 100 IU/mL penicillin and 100 g/mL streptomycin (GIBCO). Half of the culture medium was changed at day 3 to remove non-adherent cells. Subsequently, the medium was replaced entirely each week. The cells were grown for 2-3 weeks until almost confluent. Then, the adherent cells were detached with 0.25% trypsin-EDTA. Subsequent passage and cell seeding was performed at a density of 1×106 cells. BMDMSC differentiation was analyzed using flow cytometry, as described previously.13
Specific-pathogen-free, 6-week-old female BALB/c mice (Charles River Technology) were given 3% TDI dissolved in a mixture of ethyl acetate and olive oil (1:4) intranasally once daily for 5 consecutive days. Four days after sensitization, the mice were challenged with 1% TDI dissolved in a mixture of ethyl acetate and olive oil by ultrasonic nebulization for 1 hour over 3 consecutive days, according to a published method (Fig. 1).13,19 As a control group, sham mice were sensitized and challenged with only a mixture of ethyl acetate/olive oil. SD rat BMDMSCs (1×105 cells) were transferred to the TDI-induced asthma mice intravenously via tail-vein injection 1 day before the TDI challenge. The mice were killed on day 5 after BMDMSC transfer. The Institutional Animal Care and Use Committee of Soonchunhyang University approved the study.
Airway parameters were measured 4 days after the BMDMSC transfer into TDI-sensitized mice. Mice were placed in a barometric plethysmographic chamber (All Medicus, Seoul, Korea), and baseline readings were taken for 3 minutes. The enhanced pause (Penh) was calculated according to the manufacturer's protocol [i.e., (expiratory time/relaxation time - 1)×(peak expiatory flow/peak inspiratory flow)]. Penh is a dimensionless parameter that represents a function of the proportion of maximal expiratory to maximal inspiratory box pressure signals and a function of the timing of expiration.21,22 Results are expressed as the percentage increase in Penh following challenge with each concentration of methacholine (5, 20, and 50 mg/mL). Twenty-four hours after measuring the airway parameters, the mice were killed with pentobarbital sodium (65 mg/kg, i.p.), and PBS was infused slowly into the lungs and withdrawn via a cannula inserted into the trachea to obtain bronchoalveolar lavage (BAL) fluid. The cells in the BAL fluid were counted, placed on slides by cytocentrifugation, and stained using Diff-Quick (Scientific Products, Gibbstown, NJ, USA). Then, the BAL fluid was centrifuged, and the supernatant was kept at -70℃.
Trachea and lung tissues were removed from the mice. Then, 4% paraformaldehyde fixative solution was infused into the lungs via the trachea. The specimens were dehydrated and embedded in paraffin. For histological examination, 4-µm sections of embedded tissue were cut on a rotary microtome, placed on glass slides, deparaffinized, and stained sequentially with hematoxylin and eosin (H&E) and periodic acid-Schiff (PAS). The severity of peribronchial inflammation was graded semiquantitatively as previously described: 0, normal; 1, a few cells; 2, a ring of inflammatory cells one cell deep; 3, a ring of inflammatory cells more than four cells deep.19,23 We counted PAS-positive epithelial cells and all epithelial cells using software (Nikon DXM 1200; Nikon, Tokyo, Japan; and Image-Pro Plus 4.01; Media Cybernetics, Bethesda, MD, USA).
For immunohistochemistry of smooth muscle actin (SMA) and proliferating cell nuclear antigen (PCNA), lung tissue on slides was treated with 0.3% H2O2 for 30 minutes to block endogenous peroxidase and then incubated at 4℃ overnight with anti-mouse SMA goat polyclonal antibody (1:200 dilution; Santa Cruz Biotechnology, Santa Cruz, CA, USA) and anti-mouse PCNA rabbit polyclonal antibody (1:200 dilution; Santa Cruz Biotechnology). After the slides were incubated with an avidin-biotin complex kit (ABC; Vector Laboratories, Burlingame, CA, USA), color was developed with 3,3' diaminobenzidine (DAB; Zymed Laboratories, San Francisco, CA, USA).
Lung tissues were fixed in Bouin's solution, washed in tap water (5 minutes at room temperature), and then stained for 10 minutes with Weigert's iron hematoxylin. After washing in tap water, the slides were stained with a mixture of 1% acid fuchsin and 1% Biebrich scarlet in distilled water for 2 minutes and then treated with 2.5% phosphomolybdic-phosphotungstic acid for 10 minutes. Finally, the sections were stained with aniline blue for 1 minute, treated with 1% acetic acid for 1 minute, and then dehydrated in an ethanol/water series followed by five changes in absolute ethanol, cleared in xylene, and mounted in balsam. The collagen assay was performed according to the Sircol collagen assay kit manual (Biocolor, Carrickfergus, Northern Ireland, UK). Briefly, 100 µL lung tissue protein extract was mixed with 1 mL of Sircol dye for 30 minutes and centrifuged at 10,000 rpm for 5 minutes to drop the formed collagen-dye complex. After decanting the suspension, droplets were dissolved in 1 mL Sircol alkali reagent and vortexed. The acquired solution (100 µL) was read at 540 nm, as previously described.13
Data are expressed as the mean±standard error of the mean. SPSS version 10.0 (SPSS, Chicago, IL, USA) was used. Continuous data were compared using the Kruskal-Wallis test. If differences were found to be significant, the Mann-Whitney U-test was applied to compare differences between two samples. Differences were considered significant when P<0.05.
The BMDMSCs used in this study expressed CD44H and CD45 on the surface based on flow cytometry analysis, as described previously.13 TDI-sensitized/challenged mice had a significantly more cells and fractions of inflammatory cells in BAL fluid compared with sham mice (P<0.05; Fig. 2). BMDMSC transfer significantly reduced the TDI-induced increase in the number of cells, neutrophils, eosinophils, and macrophages in BAL fluid to the level of that in sham-treated mice (P<0.05; Fig. 2). On histologic examination, TDI-sensitized/challenged mice had a heavy infiltration of inflammatory cells and exudative changes in the peribronchial layers and intraluminal areas of the bronchi. BMDMSC transfer significantly reduced the cellular infiltration induced by TDI-sensitization/challenge (Fig. 3A). The semiquantitative value of the inflammatory index from H&E-stained images was significantly attenuated by the infusion of BMDMSCs in the TDI-sensitized/challenged mice (Fig. 3B).
The percentage of PAS-positive goblet cells (Fig. 3C) increased significantly in the TDI-sensitized/challenged mice, and the BMDMSC infusion significantly reduced the number of goblet cells to the sham level (P<0.0001). PCNA protein expression (Fig. 4A) markedly increased in the peribronchial muscle layer and in the epithelial cells in the bronchi of TDI-sensitized/challenged mice, and this was effectively reduced by BMDMSC transfer. TDI-sensitized/challenged mice also strongly expressed SMA protein in the bronchial and intraluminal exudations of the intrapulmonary bronchi (Fig. 4B).
To quantify collagen deposition in the lungs, Masson trichrome staining and the Sircol collagen assay were used. As shown in Fig. 5A, collagen deposition increased in the lungs from TDI-sensitized/challenged mice, and the infusion of BMDMSCs into mice reduced the TDI-induced collagen deposition. The result concurred with the finding that BMDMSC-infused mice had significantly lower levels of measurable collagen than did the TDI-sensitized/challenged group (P<0.005; Fig. 5B), reaching the normal level of the sham group.
The Penh values increased significantly in TDI-sensitized/challenged mice compared with the sham mice at 20 and 50 mg/mL methacholine (P<0.005 and P<0.01, respectively; Fig. 6). The BMDMSC infusion reduced the increased Penh values of the TDI-sensitized/challenged mice at 50 mg/mL methacholine to the level of sham controls (P<0.01; Fig. 6).
This study evaluated the effects of BMDMSCs on both inflammation and remodeling in a TDI-induced mouse asthma model. TDI is a low-molecular-weight compound used widely in the production of polyurethane foams, automobile paint, varnishes, and related products. TDI-induced asthma may reflect a subtype of severe asthma.24 Approximately 5-10% of workers in these settings develop occupational asthma with chronic airways inflammation. Therefore, we used a TDI-induced asthma mouse model as a subtype of severe asthma.
In this study, the BMDMSC infusion inhibited the TDI-induced increase in the numbers of macrophages, eosinophils, and neutrophils (Fig. 2), which suggests that BMDMSCs inhibit inflammatory cell infiltration non-selectively. Mice exposed to TDI developed features of airway remodeling, including thickening of the peribronchial smooth muscle layer, subepithelial collagen deposition, and increased airway mucus production.12 In our study, SMA and PCNA stains were used to measure myofibroblast and smooth muscle thickening and epithelial cell damage and regeneration associated with remodeling. Notably, the BMDMSC infusion effectively inhibited the increased SMA-positive muscle layer, and PCNA-positive epithelial cells induced by TDI-sensitization/challenge (Fig. 4), suggesting that BMDMSCs effectively inhibit airway remodeling in asthma. In addition, the semiquantitative value of the inflammatory index and the percentage of goblet cells induced by TDI-sensitization/challenge were significantly reduced after BMDMSC transfer, suggesting that the infusion of BMDMSCs inhibits not only airway inflammation, but also mucus metaplasia. The BMDMSC infusion significantly reduced the levels of measurable collagen compared with the TDI-sensitized/challenged group, indicating that the infusion of BMDMSCs can protect remodeling in TDI-sensitized/challenged mice.
In the lungs, many studies have reported that either whole bone marrow or BMDMSCs limited experimental lung injury and fibrosis,13,18,25 but the idea that stem cells simply supply a reservoir of new lung parenchymal cells and therefore hasten repair is inadequate to explain the effect.13 Although one can demonstrate the persistence of donor stem cells in the lungs and evident differentiation into lung cell phenotypes, the number of engrafted cells is too small to explain the protective effect sufficiently.25 Similarly, infusions of whole bone marrow protect endotoxin-induced lung injury at a level disproportionate to donor cell number found in the lungs.26 The anti-inflammatory effect of BMDMSCs may be an important contributor to the inhibitory effect of stem cell administration.27
The anti-fibrotic effect of BMDMSCs has been demonstrated in bleomycin-induced pulmonary fibrosis.13 In the inhibitory mechanism of BMDMSCs, both endogenous and exogenous stem cells contribute to the repair process.25 Homing of stem cells to injured lung is at least partly a result of the production of humoral mediators by injured, but not by normal, lung that are chemotactic for stem cells.25 We did not evaluate the mechanism of BMDMSC effectiveness, and further studies are needed to clarify whether the effect of BMDMSCs is due to BMDMSCs or to humoral mediator release.
In summary, BMDMSC transfer effectively inhibited inflammation and remodeling in a TDI-induced asthma mouse model, suggesting that BMDMSCs, which possess anti-inflammatory and anti-remodeling properties, could be useful for the treatment of inflammation and remodeling in severe asthma. To date, there has been no report on the effects of BMDMSCs on inflammation and remodeling in experimental asthma. This study is the first to establish the therapeutic effects of BMDMSCs in a TDI-induced asthma model.
Figures and Tables
 | Fig. 1Schematic diagram of the experimental protocol. Mice were sensitized by the intranasal administration of 3% toluene diisocyanate (TDI) once daily for 5 consecutive days. Bone marrow-derived mesenchymal stem cells (1×105 cells) were transferred via an intravenous route. The following day, mice were challenged via the airways with 1% TDI for 3 days with 1 hr ultrasonic nebulization each day. At day 12, airway hyperresponsiveness (AHR) to methacholine was measured; then, the mice were killed on day 13. |
 | Fig. 2Cellular profiles of bronchoalveolar lavage (BAL) fluid. Toluene diisocyanate (TDI)-sensitized/challenged mice had increased numbers of cells, macrophages, neutrophils, and eosinophils compared with sham-treated mice (*P<0.05). Bone marrow-derived mesenchymal stem cell (BMDMSC) transfer effectively reduced the increased total cell, macrophage, eosinophil, and neutrophil numbers in BAL fluid from the TDI-sensitized/challenged mice (†P<0.05). |
 | Fig. 3Microscopic findings of the intrapulmonary bronchi. (A) Histological examination of lung tissue. Lung sections were obtained from sham and toluene diisocyanate (TDI)-sensitized/challenged mice with or without bone marrow-derived mesenchymal stem cell transfer. Tissue sections were stained with periodic acid-Schiff (PAS) to determine the presence of goblet cells. Scale bar=200 µm. (B) Semiquantitative analysis of the severity of peribronchial inflammation. All of the randomly selected histological images were scored using the inflammatory index, and the mean is presented. (C) PAS-positive cells in epithelium and total epithelial cells were counted, and the percentage of PAS-positive cells was calculated. *P<0.0001, compared with the sham group; †P<0.0001, compared with the TDI group. |
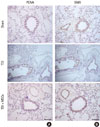 | Fig. 4Immunohistochemical staining of proteins expressed in the intrapulmonary bronchi. (A) Smooth muscle actin (SMA) protein expression increased markedly in the peribronchial muscle layer of the bronchi of toluene diisocyanate (TDI)-sensitized/challenged mice. Bone marrow-derived mesenchymal stem cell (BMDMSC) transfer reduced the expression of SMA protein. (B) Proliferating cell nuclear antigen (PCNA) protein expression increased markedly in epithelial cells in the bronchi of TDI-sensitization/challenged mice. Infusion of BMDMSCs reduced the expression of PCNA protein. A representative image from six experiments in each group is shown. Scale bar=200 µm. |
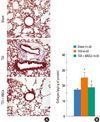 | Fig. 5Histological analysis and collagen level in the lung. (A) Masson trichrome stain in lung tissue of sham, toluene diisocyanate (TDI)-sensitized/challenged, and Bone marrow-derived mesenchymal stem cell (BMDMSC) transferred mice. Scale bar=200 µm. (B) Effects of BMDMSC transfer on collagen deposition. The total soluble collagen content in the right lung was measured using a Sircol collagen kit. Differences between the BMDMSC-infusion and TDI groups were significant. *P<0.05, compared with the sham group; †P<0.05, compared with the TDI sensitized/challenged group. |
 | Fig. 6Effect of Bone marrow-derived mesenchymal stem cell (BMDMSC) transfer on airway hyperresponsiveness to methacholine in a toluene diisocyanate (TDI)-induced murine asthma model. TDI-sensitized/challenged mice had high enhanced pause (Penh) values at 20 and 50 mg/mL methacholine (*P<0.005 vs. sham). BMDMSC transfer significantly reduced the Penh values at 50 mg/mL methacholine in TDI-sensitized/challenged mice (†P<0.01 vs. TDI). |
ACKNOWLEDGMENTS
JHK and SHL performed all of the experimental procedures and wrote the manuscript; ASJ provided experimental assistance and wrote the first draft of the manuscript; SKP and JHW isolated the BMDMSCs and evaluated the mesenchymal differentiation; CSP conceptualized the study and supervised this project. All authors read and approved the final manuscript. The authors declare that they have no competing interests.
References
1. Bateman ED, Hurd SS, Barnes PJ, Bousquet J, Drazen JM, FitzGerald M, Gibson P, Ohta K, O'Byrne P, Pedersen SE, Pizzichini E, Sullivan SD, Wenzel SE, Zar HJ. Global strategy for asthma management and prevention: GINA executive summary. Eur Respir J. 2008. 31:143–178.
2. Vignola AM, Mirabella F, Costanzo G, Di Giorgi R, Gjomarkaj M, Bellia V, Bonsignore G. Airway remodeling in asthma. Chest. 2003. 123:417S–422S.
3. American Thoracic Society. Proceedings of the ATS workshop on refractory asthma: current understanding, recommendations, and unanswered questions. Am J Respir Crit Care Med. 2000. 162:2341–2351.
4. Lange P, Parner J, Vestbo J, Schnohr P, Jensen G. A 15-year follow-up study of ventilatory function in adults with asthma. N Engl J Med. 1998. 339:1194–1200.
5. Mealey FH, Kenyon NJ, Avdalovic MV, Louie S. Difficult-to-control asthma in adults. Am J Med. 2007. 120:760–763.
6. Thomas M, Haughney J, Price D. Cost effectiveness of asthma management strategies. Pharmacoeconomics. 2002. 20:789.
7. Benayoun L, Druilhe A, Dombret MC, Aubier M, Pretolani M. Airway structural alterations selectively associated with severe asthma. Am J Respir Crit Care Med. 2003. 167:1360–1368.
8. Pepe C, Foley S, Shannon J, Lemiere C, Olivenstein R, Ernst P, Ludwig MS, Martin JG, Hamid Q. Differences in airway remodeling between subjects with severe and moderate asthma. J Allergy Clin Immunol. 2005. 116:544–549.
9. Park SW, Park JS, Lee YM, Lee JH, Jang AS, Kim DJ, Hwangbo Y, Uh ST, Kim YH, Park CS. Differences in radiological/HRCT findings in eosinophilic bronchitis and asthma: implication for bronchial responsiveness. Thorax. 2006. 61:41–47.
10. ten Brinke A, Zwinderman AH, Sterk PJ, Rabe KF, Bel EH. "Refractory" eosinophilic airway inflammation in severe asthma: effect of parenteral corticosteroids. Am J Respir Crit Care Med. 2004. 170:601–605.
11. Sont JK, Willems LN, Bel EH, van Krieken JH, Vandenbroucke JP, Sterk PJ. The AMPUL Study Group. Clinical control and histopathologic outcome of asthma when using airway hyperresponsiveness as an additional guide to long-term treatment. Am J Respir Crit Care Med. 1999. 159:1043–1051.
12. Boulet LP, Turcotte H, Laviolette M, Naud F, Bernier MC, Martel S, Chakir J. Airway hyperresponsiveness, inflammation, and subepithelial collagen deposition in recently diagnosed versus long-standing mild asthma. Influence of inhaled corticosteroids. Am J Respir Crit Care Med. 2000. 162:1308–1313.
13. Lee SH, Jang AS, Kim YE, Cha JY, Kim TH, Jung S, Park SK, Lee YK, Won JH, Kim YH, Park CS. Modulation of cytokine and nitric oxide by mesenchymal stem cell transfer in lung injury/fibrosis. Respir Res. 2010. 11:16.
14. Deans RJ, Moseley AB. Mesenchymal stem cells: biology and potential clinical uses. Exp Hematol. 2000. 28:875–884.
15. Bruder SP, Kurth AA, Shea M, Hayes WC, Jaiswal N, Kadiyala S. Bone regeneration by implantation of purified, culture-expanded human mesenchymal stem cells. J Orthop Res. 1998. 16:155–162.
16. Pereira RF, O'Hara MD, Laptev AV, Halford KW, Pollard MD, Class R, Simon D, Livezey K, Prockop DJ. Marrow stromal cells as a source of progenitor cells for nonhematopoietic tissues in transgenic mice with a phenotype of osteogenesis imperfecta. Proc Natl Acad Sci U S A. 1998. 95:1142–1147.
17. Krause DS, Theise ND, Collector MI, Henegariu O, Hwang S, Gardner R, Neutzel S, Sharkis SJ. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell. 2001. 105:369–377.
18. Ortiz LA, Gambelli F, McBride C, Gaupp D, Baddoo M, Kaminski N, Phinney DG. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci U S A. 2003. 100:8407–8411.
19. Ahn MH, Park BJ, Kwon JH, An SH, Park JW, Jang AS, Rhim T, Park CS. Asp-Tyr-Leu-Lys tetrapeptide inhibits airway inflammation in toluene-2,4-diisocyanate-induced asthma mice. Clin Exp Allergy. 2008. 38:1025–1032.
20. Anjos-Afonso F, Siapati EK, Bonnet D. In vivo contribution of murine mesenchymal stem cells into multiple cell-types under minimal damage conditions. J Cell Sci. 2004. 117:5655–5664.
21. Hamelmann E, Schwarze J, Takeda K, Oshiba A, Larsen GL, Irvin CG, Gelfand EW. Noninvasive measurement of airway responsiveness in allergic mice using barometric plethysmography. Am J Respir Crit Care Med. 1997. 156:766–775.
22. Shin YS, Takeda K, Gelfand EW. Understanding asthma using animal models. Allergy Asthma Immunol Res. 2009. 1:10–18.
23. Choi JM, Ahn MH, Chae WJ, Jung YG, Park JC, Song HM, Kim YE, Shin JA, Park CS, Park JW, Park TK, Lee JH, Seo BF, Kim KD, Kim ES, Lee DH, Lee SK. Intranasal delivery of the cytoplasmic domain of CTLA-4 using a novel protein transduction domain prevents allergic inflammation. Nat Med. 2006. 12:574–579.
24. Chan-Yeung M. Occupational asthma. Chest. 1990. 98:148S–161S.
25. Rojas M, Xu J, Woods CR, Mora AL, Spears W, Roman J, Brigham KL. Bone marrow-derived mesenchymal stem cells in repair of the injured lung. Am J Respir Cell Mol Biol. 2005. 33:145–152.
26. Yamada M, Kubo H, Kobayashi S, Ishizawa K, Numasaki M, Ueda S, Suzuki T, Sasaki H. Bone marrow-derived progenitor cells are important for lung repair after lipopolysaccharide-induced lung injury. J Immunol. 2004. 172:1266–1272.
27. Xu J, Woods CR, Mora AL, Joodi R, Brigham KL, Iyer S, Rojas M. Prevention of endotoxin-induced systemic response by bone marrow-derived mesenchymal stem cells in mice. Am J Physiol Lung Cell Mol Physiol. 2007. 293:L131–L141.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download