Abstract
Purpose
To determine the role of plasmacytoid dendritic cells (pDC) and myeloid dendritic cells (mDC) in priming effector T cells to induce allergy, and to evaluate the effect of immunostimulatory sequences (ISS, TLR9 agonist) on dendritic cells.
Methods
Cultured mDC and pDC with/without ISS were injected intratracheally into sensitized Balb/C mice. Mice were sacrificed, and then pulmonary function tests, bronchoalveolar lavage (BAL), cell counts, and cytokine levels were evaluated. Migration of dendritic cells was also evaluated after ISS administration.
Results
In mice injected with mDC, airway hyperresponsiveness, eosinophil counts, and Th2 cytokine levels in BAL increased with increasing numbers of mDC injected. However, in mice injected with pDC, none of these changed, suggesting poor priming of T cells by pDC. In addition, mDC pulsed with ISS inhibited asthmatic reactions, and ISS administration inhibited migration of DC to the lung.
Dendritic cells (DC) are antigen-presenting cells (APC) that play a crucial role in both innate and adaptive immunity, and provide antigen to T cells to induce immune responses. DC subsets include conventional and plasmacytoid DC. Conventional DC can also be divided into a number of subsets according to their localization. Myeloid dendritic cells (mDC)-a typical conventional DC subtype-can be differentiated in vitro in the presence of GM-CSF and are usually CD11c+CD11b+B220 cells.
Plasmacytoid dendritic cells (pDC, also known as interferon-producing cells [IPC]), another subset of DC, possess TLR9 receptors and can be differentiated from bone marrow-derived cells in the presence of Flt3 ligand. When stimulated with immunostimulatory sequences (ISS, TLR9 agonist), pDC secrete abundant type 1 interferon, producing anti-viral immunity.
It is well known that mDC play a major role in antigen presentation and in priming T cells. mDC are professional APC and mDC pulsed with ovalbumin (OVA) can induce asthma in the mouse model by playing a central role in orchestrating the asthmatic reaction. However, the effect of pDC on induction of asthma has not been studied extensively.
Asthma is a chronic airway disorder characterized by reversible airway obstruction, airway hyperresponsiveness, and cellular inflammation, predominantly eosinophils and allergen-specific Th2 lymphocytes.1,2 Th2 cells are the principal effector cells that coordinate many of the actions of other inflammatory cells in the airway by the release of cytokines and chemokines.3-5 We have shown that ISS inhibits allergic asthmatic reactions via various immune mechanisms, and investigations into clinical applications are underway.6 Here, we treated DC with ISS to assess the effect of ISS on asthma induction, and determined the effect of administering ISS to mice on pulmonary DC migration. We also evaluated the induction of allergic asthmatic reactions by mDC and pDC and the in vivo effect of ISS on DC migration to the lung.
Balb/c mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). All animal procedures followed the University of California, San Diego, animal-care guidelines. All experiments were performed in at least triplicate.
ISS-ODN (5'-TGACTGTGAACGTTCGAGATGA-3') was purchased from Trilink Biotechnologies (San Diego, CA, USA). OVA was purchased from Sigma-Aldrich (Saint Louis, MS, USA).
Myeloid bone marrow-derived dendritic cells (BMDC) were prepared from Balb/c mice as described previously.7 Briefly, bone marrow from the femurs and tibias of Balb/c mice was plated in petri dishes (Fisher Scientific, Pittsburgh, PA, USA) on day 0 at 2×105 cells/mL in DC medium, which consisted of RPMI (Irvine Scientific, Irvine, CA, USA) supplemented with 10% heat-inactivated FCS (Life Technologies, Gaithersburg, MD, USA), 2 mM L-glutamine (Cellgro, Natham, VA, USA), and 100 U/mL penicillin/100 µg/mL streptomycin (Pen/Strep; Cellgro) containing 5 ng/mL recombinant murine GM-CSF (BD PharMingen, San Hose, CA, USA). On day 3, an equal volume of DC medium was added. On day 6, one-half of the volume of DC medium was replaced. Non-adherent cells were harvested on day 7. For plasmacytoid dendritic cells, bone marrow from the femurs and tibias of Balb/c mice was plated in petri dishes (Fisher Scientific, Pittsburgh, PA, USA) on day 0 at 1.5×106 cells/mL in DC medium containing 5 ng/mL recombinant human Flt3 ligand (Peprotech, Rocky Hill, NJ, USA). Non-adherent cells were harvested on day 14. Further purification of pDC cells using anti-CD11c magnetic beads, B220 magnetic beads, and an MACS column (Miltenyi Biotec, Auburn, CA, USA) as per the manufacturer's instructions was performed for selected studies. Flow cytometry, after staining with Abs against the following cell surface markers or appropriate isotype controls (BD PharMingen), was conducted to characterize the cultured cells: CD11c (clone HL3), CD11b (clone M1/70), and B220 (clone RA3-6B2). Single-cell lung suspensions were prepared by collagenase digestion as described previously.8 The DC population was initially enriched by discontinuous density gradient centrifugation using OptiPrep (Axis-Shield PoC AS, Oslo, Norway) according to the manufacturer's instructions. Low-density cells at the interface were harvested, and CD11c+ cells were positively isolated using anti-CD11c magnetic beads (Miltenyi Biotec Inc., Auburn, CA, USA). The CD11c+ population comprised greater than 90% of the total cells as determined by FACS analysis. High-density cells were collected and served as a CD11c-cell population.
Airway inflammation was induced according to a protocol previously described with minor modifications.9 In brief, Balb/c mice were sensitized by subcutaneous injection of 20 µg OVA absorbed with 500 µg alum (Alhydrogel; Brenntag Biosector AS, Federikssund, Denmark) per mouse in saline on days 0, 7, and 14. On days 20 and 25, mice were challenged intratracheally with 106 DC pulsed with or without OVA (Figs. 1A, 2A) or intranasally with 5 µg OVA (Fig. 3A). Mice were then sacrificed on day 26.
To evaluate the role of mDC, mice were divide into groups (n=3) and 105, 3×105, or 106 mDC pulsed with OVA were instilled intratracheally (Fig. 1A). Next, mice were divided into six groups to evaluate the roles of mDC and pDC with or without ISS. mDC alone (group 1), mDC pulsed with OVA (group 2), mDC pulsed with OVA and ISS (group 3), pDC alone (group 4), pDC pulsed with OVA (group 5), or pDC pulsed with OVA and ISS (group 6) were administered intratracheally on days 20 and 25. One million pDC or mDC were administered to each mouse (Fig. 2A). mDC and pDC were pulsed with Ova and/or ISS for 30 min and were washed thoroughly before instillation.
In the final experiment, mice were divided into four groups: group 1 (control, no sensitization), group 2 (sensitization with OVA and alum, PBS challenge), group 3 (sensitization with OVA and alum, OVA challenge, no treatment), and group 4 (sensitization with OVA and alum, OVA challenge, ISS treatment). After sensitization with OVA and alum, ISS-ODN (50 µg/mouse) was administered intraperitoneally 1 day before OVA challenge (day 19).
Twenty-four hours after the last challenge (day 26), mice were subjected to airway responsiveness tests and then sacrificed by CO2 asphyxiation. Five mice were used in each group, and measurements were taken in triplicate.
After sacrifice, bronchoalveolar lavage (BAL) cells and lungs were harvested from each mouse. The total number of cells in BAL was counted, and BAL cells were stained with Wright-Giemsa stain for the differential count. Percentages of eosinophils were determined microscopically using standard morphological criteria.10 The total number of eosinophils was calculated from the total cell number and the percentage of eosinophils in the differential cell count. The levels of each cytokine (IL-4, IL-5, IFN-γ) in BAL fluid were determined by ELISA (BD Biosciences - Pharmingen, San Diego, CA, USA).
Airway responsiveness to methacholine (MCh) was assessed 24 hours after the final OVA challenge using a single-chamber, whole-body plethysmograph obtained from Buxco Electronics Inc. (Wilmington, NC, USA), as described previously.9 The Penh, a dimensionless value, which correlates well with pulmonary resistance measured by conventional two-chamber plethysmography in ventilated mice, was used to monitor airway responsiveness. In the plethysmograph, mice were exposed for 3 minutes to nebulized PBS to establish baseline Penh values and were subsequently exposed to increasing concentrations of nebulized MCh (Sigma-Aldrich) in PBS using an Aerosonic ultrasonic nebulizer (DeVilbiss Health Care Inc., Somerset, PA, USA). Following each nebulization, recordings were taken for 3 minutes. The Penh values measured during each 3-minute sequence were averaged and are expressed for each MCh concentration as the percentage of baseline Penh values following PBS exposure.
To elucidate the effect of mDC on asthma induction, 105, 3×105, or 106 mDC pulsed with OVA were administered intratracheally (Fig. 1A). mDC induced airway hyperresponsiveness in an mDC number-dependent manner; the difference between groups was statistically significant (P=0.002; Fig. 1B). Eosinophil counts in BAL fluid increased significantly with increasing numbers of mDC (each P<0.05; Fig. 1C). Th2 cytokine (IL-4 and IL-5) levels in BAL also increased significantly and interferon-γ decreased, with increasing mDC administration (Fig. 1D).
To evaluate the effect of pDC on asthma, mDC or pDC (106) with or without OVA were administered intratracheally after sensitization of mice with OVA and alum. To assess the effect of ISS on DC, mDC and pDC were also incubated with ISS prior to intratracheal administration (groups 3 and 6). The experimental protocol is illustrated in Fig. 2A. pDC were isolated using anti-CD11c and anti-B220 MACS beads, and CD11c+ and B220+ groups were isolated with a purity of 94.4% (Fig. 2B). Airway hyperresponsiveness and eosinophil counts induced by mDC (group 2) were abolished with mDC pulsed with ISS administration (group 3; P=0.01 and P<0.001, respectively; Fig. 2C, D). Th2 cytokine levels also significantly decreased in the ISS-pulsed, mDC-administered group (all P<0.01). However, in the pDC-administered groups (groups 4, 5, and 6), airway hyperresponsiveness and eosinophil counts did not differ from those of the non-OVA-pulsed mDC-administered group (group 1), suggesting that priming of T cells by pDC with OVA antigen is poor. IL-4 and IL-5 levels were not significantly different compared to group 1 (all P>0.05).
We also evaluated the in vivo effect of ISS on pulmonary DC populations. ISS was administered to mice on day 19 (Fig. 3A) and the DC population was counted using flow cytometry. The CD11c+CD11b+ cell count increased from 1.84% to 15.80% after OVA challenge and decreased to 9.02% in the ISS-administration group, showing that ISS inhibited migration of mDC populations to the lungs. The proportion of CD11c+B220+ cells increased from 0.60% to 1.08% after OVA challenge, and decreased to 0.69% after ISS administration. In addition, ISS also significantly decreased eosinophil counts and Th2 cytokine (IL-4 and IL-5) levels in the lungs (all P<0.01; Fig. 3C). Interferon-γ levels were not significantly different after ISS administration (P>0.05; Fig. 3C).
The present study demonstrated that mDC are potent APC that orchestrate the asthmatic reaction, and that pDC do not elicit an asthmatic reaction in a mouse model, whether treated with ISS or not.
pDC have a plasma cell-like morphology and were recently identified in lymphoid tissues as CD11c+B220+Gr-1+CD45Rbhigh-CD11b- cells.11,12 Although the mechanism and regulation of pDC development is not clearly understood, pDC can be grown with the aid of the Flt3 ligand, which is the only known cytokine to promote pDC development; its effect has been confirmed in human in vivo studies. We used Flt3 ligand to culture pDC in vitro and isolated them using anti-CD11c and anti-B220 MACS antibodies. Although the isolated pDC were not 100% pure, their purity was about 94%. Thus, these CD11c+B220+ pDC well represent the general pDC population.
We first confirmed the capacity of mDC to elicit an asthmatic reaction using increasing numbers of mDC pulsed with OVA. Intratracheally administered mDC induced asthma in an mDC number-dependent manner. As the numbers of mDC administered intratracheally increased, airway hyperresponsiveness, eosinophil counts, and Th2 cytokines in BAL also increased, suggesting an important role of mDC in initiating and orchestrating asthmatic reactions.
In contrast to mDC, pDC have been reported to be only poorly phagocytic and pinocytic.13 pDC can present endogenous viral antigen and prime CD8 T cell responses to endogenous antigens or peptides, but not to exogenous antigens,14 implying greater specialization in presenting viral or endogenous antigens. Exogenous OVA antigen can be taken up by both pDC and mDC; however, only mDC can induce the generation of effector T cells,15 which is consistent with our data that pDC pulsed with OVA did not induce asthma in mice. A previous study reported that adoptive transfer of pDC before sensitization inhibited asthmatic reactions, and pDC suppressed the generation of effector T cells.15 We also used a mixture of pDC and mDC, expecting to see inhibition of asthmatic reactions by pDC. However, no such inhibition was detected (data not shown). The variation in injection time may have caused this difference.
We showed that ISS-treated mDC inhibited asthmatic reactions (Fig. 2), implying that ISS may alter the ability of mDC to inhibit Th2 response. ISS-treated mDC are known to change from a pro- to an anti-inflammatory phenotype in the mouse colitis model,16 implying that such a change in DC phenotype caused by ISS may have occurred in this case. The anti-asthmatic effect of ISS has been reported many times, and here we demonstrated that mDC pulsed with ISS changed the cytokine milieu of the lungs (Fig. 2D). Additionally, ISS significantly inhibited migration of DC to the lungs, suggesting an anti-asthmatic mechanism of ISS (Fig. 3). Although the population of CD11c+B220+ cells also decreased from 1.08% to 0.69% after ISS administration, this proportion of CD11c+B220+ cells is so small that the meaning of these data should be evaluated further.
In conclusion, we found that pDC played only a limited role in priming T cells in the mouse model of asthma using OVA antigen; however, mDC played a major role in the induction of asthma. In addition, ISS had inhibitory effects on DC, inducing asthma by inhibiting migration of DC to the lungs.
Figures and Tables
Fig. 1
Myeloid dendritic cells (DC) induced an asthmatic reaction in myeloid dendritic cells (mDC) in a number-dependent manner. (A) Schematic illustration of the experimental protocol. Different numbers of myeloid DC were administered intratracheally to Balb/c mice after ovalbumin/alum sensitization. Five mice were used in each group and this experiment was performed in triplicate. (B) Airway hyperresponsiveness to methacholine was evaluated. mDC increased airway hyperresponsiveness in a number-dependent manner. (C) Eosinophil counts in bronchoalveolar lavage (BAL) fluid also depended on the number of mDC administered. (D) IL-4 and IL-5 levels increased with increasing numbers of mDC in BAL fluid. Interferon-γ decreased in group 3. Gp, group; IFN- γ, interferon-γ; MCh: methacholine.

Fig. 2
Isolation of plasmacytoid dendritic cells (DC) and the effect of plasmacytoid dendritic cells (pDC) with or without an immunostimulatory sequence (ISS) on asthma induction. (A) Schematic illustration of the experimental protocol. Myeloid dendritic cells (mDC) or pDC (106) with or without ISS stimulation were administered intratracheally to Balb/c mice after ovalbumin (OVA)/alum sensitization. Five mice were used in each group and this experiment was performed in duplicate. (B) Characterization of pDC subsets after isolation using anti-CD11c and anti-B220 MACS antibodies. Isolated cells exhibited 94.4% purity. (C, D) Airway hyperresponsiveness curve showing that pDC with or without OVA pulsing did not induce airway hyperresponsiveness compared to mDC without OVA (P>0.05). mDC pulsed with OVA increased airway hyperresponsiveness, eosinophil counts, and IL-4 and IL-5 levels in BAL, the effect of which was abolished by administration of mDC pulsed with ISS and OVA.
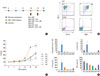
Fig. 3
Immunostimulatory sequence (ISS) inhibits myeloid dendritic cells (mDC) migration into the lung of mice in vivo. (A) Schematic illustration of the experimental protocol. Mice were sensitized with ovalbumin (OVA)/alum and ISS was administered intraperitoneally on day 19. Intranasal OVA challenge was performed on days 20 and 25, and this experiment was performed in duplicate. (B) CD11c+CD11b+ cell counts increased from 1.84% to 15.80% after OVA challenge and decreased to 9.02% in the ISS administration group. In the case of CD11c+B220+ cells, counts increased from 0.60% to 1.08% after OVA challenge, and then decreased to 0.69% after ISS administration. (C) ISS administration significantly lowered eosinophil counts and Th2 cytokine (IL-4 and IL-5) levels in BAL fluid (all, P<0.01).

References
1. Drazen JM, Arm JP, Austen KF. Sorting out the cytokines of asthma. J Exp Med. 1996. 183:1–5.
2. Robinson DS, Hamid Q, Ying S, Tsicopoulos A, Barkans J, Bentley AM, Corrigan C, Durham SR, Kay AB. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med. 1992. 326:298–304.
3. Corry DB, Folkesson HG, Warnock ML, Erle DJ, Matthay MA, Wiener-Kronish JP, Locksley RM. Interleukin 4, but not interleukin 5 or eosinophils, is required in a murine model of acute airway hyperreactivity. J Exp Med. 1996. 183:109–117.
4. Garlisi CG, Falcone A, Billah MM, Egan RW, Umland SP. T cells are the predominant source of interleukin-5 but not interleukin-4 mRNA expression in the lungs of antigen-challenged allergic mice. Am J Respir Cell Mol Biol. 1996. 15:420–428.
5. Lambrecht BN, De Veerman M, Coyle AJ, Gutierrez-Ramos JC, Thielemans K, Pauwels RA. Myeloid dendritic cells induce Th2 responses to inhaled antigen, leading to eosinophilic airway inflammation. J Clin Invest. 2000. 106:551–559.
6. Kanzler H, Barrat FJ, Hessel EM, Coffman RL. Therapeutic targeting of innate immunity with Toll-like receptor agonists and antagonists. Nat Med. 2007. 13:552–559.
7. Lutz MB, Kukutsch N, Ogilvie AL, Rössner S, Koch F, Romani N, Schuler G. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999. 223:77–92.
8. Vermaelen KY, Carro-Muino I, Lambrecht BN, Pauwels RA. Specific migratory dendritic cells rapidly transport antigen from the airways to the thoracic lymph nodes. J Exp Med. 2001. 193:51–60.
9. Broide D, Schwarze J, Tighe H, Gifford T, Nguyen MD, Malek S, Van Uden J, Martin-Orozco E, Gelfand EW, Raz E. Immunostimulatory DNA sequences inhibit IL-5, eosinophilic inflammation, and airway hyperresponsiveness in mice. J Immunol. 1998. 161:7054–7062.
10. Broide DH, Stachnick G, Castaneda D, Nayar J, Miller M, Cho JY, Roman M, Zubeldia J, Hayashi T, Raz E. Systemic administration of immunostimulatory DNA sequences mediates reversible inhibition of Th2 responses in a mouse model of asthma. J Clin Immunol. 2001. 21:175–182.
11. Asselin-Paturel C, Boonstra A, Dalod M, Durand I, Yessaad N, Dezutter-Dambuyant C, Vicari A, O'Garra A, Biron C, Brière F, Trinchieri G. Mouse type I IFN-producing cells are immature APCs with plasmacytoid morphology. Nat Immunol. 2001. 2:1144–1150.
12. Nakano H, Yanagita M, Gunn MD. CD11c(+) B220(+) Gr-1(+) cells in mouse lymph nodes and spleen display characteristics of plasmacytoid dendritic cells. J Exp Med. 2001. 194:1171–1178.
13. Grouard G, Rissoan MC, Filgueira L, Durand I, Banchereau J, Liu YJ. The enigmatic plasmacytoid T cells develop into dendritic cells with interleukin (IL)-3 and CD40-ligand. J Exp Med. 1997. 185:1101–1111.
14. Rissoan MC, Soumelis V, Kadowaki N, Grouard G, Briere F, de Waal Malefyt R, Liu YJ. Reciprocal control of T helper cell and dendritic cell differentiation. Science. 1999. 283:1183–1186.
15. de Heer HJ, Hammad H, Soullié T, Hijdra D, Vos N, Willart MA, Hoogsteden HC, Lambrecht BN. Essential role of lung plasmacytoid dendritic cells in preventing asthmatic reactions to harmless inhaled antigen. J Exp Med. 2004. 200:89–98.
16. Abe K, Nguyen KP, Fine SD, Mo JH, Shen C, Shenouda S, Corr M, Jung S, Lee J, Eckmann L, Raz E. Conventional dendritic cells regulate the outcome of colonic inflammation independently of T cells. Proc Natl Acad Sci U S A. 2007. 104:17022–17027.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download