Abstract
Purpose
With the increase in vancomycin use, adverse drug reactions (ADRs) associated with vancomycin have been reported increasingly more often. However, the characteristics of cutaneous ADRs with and without systemic reactions (SRs) have not been described. This study investigated the characteristics of spontaneously reported and assessed ADRs associated with vancomycin by a pharmacovigilance center.
Methods
ADRs (n=121) associated with vancomycin in 96 patients were collected from 2008 to 2009. Records from physician- and nurse-reported suspected cases of vancomycin ADRs, ADR type, latent period, and laboratory results were compared between cutaneous ADRs with and without SRs.
Results
The main vancomycin-related ADRs were skin rashes (47.9%), hematologic abnormalities (17.36%), fever (12.4%), and elevated serum creatinine (12.4%). Significant differences were observed in latent period (days) and the mean change in eosinophils (%) between cutaneous (9.21±9.71 and 1.4±3.4, respectively) and other ADRs (14.03±11.71 and -0.5±3.5, respectively). Twelve cases of cutaneous ADRs with SRs had been initially reported as cutaneous ADRs only. Mean changes in the eosinophil count were significantly higher for cutaneous ADRs with SRs compared to those without SRs.
Conclusions
Skin rashes accompanied by peripheral eosinophilia, representing suspected immune-mediated delayed hypersensitivity reactions, are a common vancomycin ADR. For the early and exact detection of ADRs associated with vancomycin administration, close monitoring of laboratory tests, including complete blood counts with differential analysis, is recommended.
Vancomycin is a complex tricyclic glycopeptide antibiotic obtained from the Nocardia species Amycolatopsis orientalis. As it has strong bactericidal activity against a broad range of Gram-positive bacteria,1 vancomycin has been used for more than 50 years and is the drug of choice for the treatment of infections due to methicillin-resistant Staphylococcus aureus (MRSA), Corynebacterium jeikeium, resistant strains of Streptococcus pneumoniae, and pseudomembranous colitis. Vancomycin is also an alternative drug for people with allergies to penicillins and/or cephalosporins.2,3
With the increase in vancomycin use, numerous adverse drug reactions (ADRs) have been reported, including red man syndrome (RMS), which is an infusion-related reaction peculiar to vancomycin. A pruritic, erythematous rash involving the face, neck, and upper torso is another typical ADR. Less frequently, vasculitis, anaphylaxis,4 ototoxicity, neutropenia, fixed drug eruptions, fever, phlebitis, nephrotoxicity,5 thrombocytopenia,6 and, more rarely, Stevens-Johnson or drug rash with eosinophilia and systemic symptoms (DRESS) syndrome have been reported.7 A recent study conducted at six Korean pharmacovigilance centers (PVCs) reported that antibiotics including vancomycin were the most prevalent causes of ADRs, and that skin manifestations were the most common symptoms in spontaneously reported ADRs.8 Of the 1,418 cases in the study, 3.1% were associated with vancomycin.
While cutaneous ADRs are common, the characteristics that distinguish cutaneous ADRs with systemic reactions (SRs) from those without SRs have not been reported. The present study was designed to investigate and compare the characteristics of cutaneous ADRs with and without SRs associated with vancomycin.
ADRs associated with vancomycin were detected at Ajou University Medical Center between January 2008 and December 2009. An electronic reporting system linked to the order communication system was used by the physicians and nurses to report ADRs. Spontaneously reported cases were reviewed and assessed by members of the PVC concerning causalities and outcomes. After the ADRs were classified according to World Health Organization-Uppsala Monitoring Center (WHO-UMC) criteria, unlikely or un-assessable cases were excluded from further analysis. The characteristics of the cutaneous ADRs were compared with the others in terms of demographic data, latent period (i.e., days from the start of vancomycin use to development of the ADR), and laboratory abnormalities. The cutaneous ADRs were further classified into two groups depending on whether they were accompanied by a SR. Fever, gastrointestinal discomfort, hematologic abnormalities, abnormal liver function, and elevated serum creatinine were classified as SRs. The two groups were also compared in terms of their laboratory test results, with an emphasis on a complete blood count with differential, and on indicators of liver and kidney function, including aspartate transaminase (AST), alanine transaminase (ALT), blood urea nitrogen, creatinine (Cr), and the level of vancomycin in some cases. Eosinophilia was defined as an absolute eosinophil count that increased by more than 500/µL or a >10% increase in the total white blood count (WBC) in cases in which the baseline level was within the normal range. Serum vancomycin concentrations were determined by a fluorescence polarization immunoassay using a Cobas Integra 800 apparatus (Roche Diagnostics, Mannheim, Germany). Pharmacokinetic parameters were obtained by the Bayesian method using CAPCIL (Simkin, Gainsville, FL, USA).
The data were analyzed using SPSS version 12.0 (SPSS Inc., Chicago, IL, USA). All descriptive statistics are presented as the frequency and mean±standard deviation. Statistical significance was evaluated using a t-test. A P-value <0.05 was regarded as statistically significant.
A total of 121 ADRs associated with vancomycin was reported in 96 patients during the study period (Table 1). Overall, the mean age of the patients (57 men and 39 women) was 48.5±17.8 years (range 12-90 years). The clinical department reporting ADRs most frequently was neurosurgery (18 cases), followed by orthopedics (17 cases), pulmonology (11 cases), and hemato-oncology (10 cases). Most cases were probable/likely (37 cases, 38.5%) and possible (53 cases, 55.2%) based on WHO-UMC criteria. More than 80% of the vancomycin-associated ADRs were reported by physicians. The mean latent period was 11.1 days.
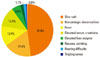
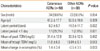
The main vancomycin-related ADRs were skin rashes (58 cases, 47.9%), hematologic abnormalities (21 cases, 17.4%), fever (15 cases, 12.4%), elevated serum Cr (15 cases, 12.4%), and liver function test abnormalities (7 cases, 5.8%). Nausea (2 cases, 1.7%) and hearing difficulties (2 cases, 1.7%) were reported rarely (Figure). The mean latent period for cutaneous ADRs was 9.2 days, which is significantly shorter than the 14-day mean latent period for the other ADRs (P=0.032, Table 2). Other than that, the mean change in eosinophils (%) was significantly different between the cutaneous and other ADRs (P=0.033, Table 2). Furthermore, 29% of the cutaneous ADR cases were reported as ADRs detected only on the day of vancomycin administration. While one case (2.6%) of nausea and vomiting was reported immediately after vancomycin administration, the rest of the ADRs were identified several days after vancomycin use. No significant difference was detected in the mean age, sex, or therapeutic drug monitoring level between the patients with a cutaneous ADR and those in the other ADR group (Table 2).
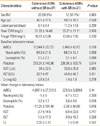
Among the 58 cutaneous ADR cases, 9 were reported as manifesting a SR such as leukopenia, thrombocytopenia, and elevated liver enzymes or Cr; the remaining 49 cases manifested only cutaneous ADRs. Notably, a difference in the number of cutaneous ADRs with systemic reactions was observed between the spontaneously reported results and reviewed results. Twelve cases of cutaneous ADRs with SRs were reported as skin rashes. Ten cases of eosinophilia, 8 of elevated liver enzymes, 4 of cytopenia, and 2 of elevated creatinine were missed during spontaneous reporting. Therefore, we ultimately compared 37 cases of cutaneous ADRs without SRs and 21 cases with SRs according to the demographic data, latent period, and laboratory test results, including the peak and trough therapeutic drug monitoring levels, complete blood count with differential, AST, ALT, and Cr. No significant differences were observed in age and sex distribution between the two patient groups. The baseline complete blood count with differential and liver enzyme and Cr values showed no significant differences. Furthermore, regarding the mean change in laboratory values, only the percentage of eosinophils was significantly higher in patients who had cutaneous ADRs with SRs compared to those without SRs (3.6±4.6 vs. 0.2±1.7%, respectively; P=0.018, Table 3).
Antibiotic ADRs are not a rare occurrence among hospitalized patients, irrespective of their age. In prospective trials, treatment-related adverse reactions were observed in 22-43% of patients in vancomycin arms.9,10 The clinical manifestations of an antibiotic allergy may be cutaneous, organ-specific (e.g., hematologic abnormalities, hepatitis, and nephritis), or systemic (e.g., anaphylaxis, fever, or a combination).11 Among them, skin rashes comprised about 5% of the ADRs.
The present study focused on cutaneous ADRs induced by vancomycin among spontaneously reported ADRs at a university hospital. In particular, to discriminate cutaneous ADRs with and without SRs, PVC teams evaluated both the reported data from medical personnel and changes in the patients' laboratory results, including their blood counts, liver and renal function tests, and therapeutic vancomycin monitoring levels before and after the ADR. Of 58 cutaneous ADR cases (36.2%), 21 simultaneously experienced SRs. Among them, 10 cases had newly developed eosinophilia with or without other manifestations after the administration of vancomycin. The mean change in eosinophil count was significantly higher in cutaneous ADRs with SRs compared to those without SRs. This suggests that at least 17% (10 cases) of the spontaneously reported cutaneous ADRs (58 cases) due to vancomycin were immune-mediated, delayed hypersensitivity reactions.
To date, the most common vancomycin-related ADR is a cutaneous adverse reaction (i.e., RMS). RMS has been reported in 3.7-50% of infected patients and up to 90% of healthy volunteers.12-14 It is thought to be the result of non-immunologic mast cell degranulation15 and histamine release following a rapid infusion of vancomycin. Therefore, RMS can be prevented by decreasing the rate or dose of infused vancomycin and by premedication with antihistamines. Vancomycin-induced anaphylaxis, an IgE-mediated immediate hypersensitivity reaction, is rare but does occur, and the readministration of vancomycin may cause bronchospasm or collapse in affected patients.4 Delayed hypersensitivity reactions caused by vancomycin can be manifested as DRESS syndrome, which is characterized by a skin rash, fever, eosinophilia, and visceral involvement (e.g., interstitial nephritis and hepatitis).7,16-19 In our study, two patients showed a skin rash, fever, and eosinophilia, but no visceral involvement occurred in either case. In another patient diagnosed initially with DRESS syndrome, eosinophilia was aggravated during vancomycin use. In a previous study, vancomycin skin tests could not predict the severity of RMS20; however, more recently developed skin tests, including intradermal and patch tests, may be useful in predicting glycopeptide-induced cutaneous adverse reactions.7,21
While 29.3% of the 58 patients with a cutaneous ADR were found to have rashes on the day of vancomycin use in this study, the mean latent period was longer than a week in both the cutaneous and other ADR groups. Although the results of a secondary challenge with vancomycin were unknown in most cases, in general, cutaneous ADRs occurring several days or weeks after exposure to the suspected medications are suggestive of immune-mediated hypersensitivity reactions. The present study indicates that increased suspicion of systemic manifestations and a more thorough investigation of laboratory results in cases of cutaneous ADRs may help detect cutaneous ADRs with SRs earlier and reduce unnecessary hospitalization caused by ADRs.
ADRs related to vancomycin may be synergistic with other drugs. Both vancomycin and narcotics induce dose- or rate-dependent mast cell degranulation22 and likely synergize to produce adverse reactions.1 Muscle relaxants23 and radiocontrast media may induce mast cell degranulation; thus, synergism may be observed when they are used simultaneously with vancomycin. Most of these cases were reported from the departments of orthopedics and neurosurgery, where the use of narcotics, muscle relaxants, and radiocontrast media is more prevalent compared to other departments. However, the concomitant use of narcotics or muscle relaxants in cutaneous ADRs with SRs did not exceed that in patients with cutaneous ADRs without SRs.
To investigate whether the serum vancomycin concentration could influence the development of ADRs, the results of therapeutic vancomycin monitoring were reviewed in 51 patients. Vancomycin has been shown to degranulate cutaneous mast cells and cause a dose-dependent area of flare in suspected patients and healthy volunteers.20 However, in the current patient comparison, no difference was detected in the peak and trough levels of vancomycin therapeutic dose monitoring in a comparison of cutaneous and other ADRs, and between cutaneous ADRs with and without SRs.
This study based on spontaneously reported ADRs in a university hospital has certain limitations. The prevalence of vancomycin-induced cutaneous ADRs was not estimated and the documentation of skin rashes varied. However, we identified 12 more cases of cutaneous ADRs with SRs among the 58 reported cases of skin rashes, and one-third of the patients with a vancomycin-related cutaneous ADR may have had more severe reactions (e.g., internal organ involvement).
With the increasing use of vancomycin due to the emergence of MRSA and resistant coagulase-negative staphylococci, a high index of suspicion and rapid, exact diagnosis are necessary. We suggest that close monitoring of a patient's laboratory values, including a complete blood count with eosinophils, may facilitate the detection of cutaneous ADRs with SRs. In addition, health care professionals should be cautious of SRs and skin rashes during vancomycin treatment.
Figures and Tables
ACKNOWLEDGMENTS
This research was supported by a 2010 grant (09182KFDA847) from the Korean Food & Drug Administration.
References
1. Wong JT, Ripple RE, MacLean JA, Marks DR, Bloch KJ. Vancomycin hypersensitivity: synergism with narcotics and "desensitization" by a rapid continuous intravenous protocol. J Allergy Clin Immunol. 1994. 94:189–194.
2. Sivagnanam S, Deleu D. Red man syndrome. Crit Care. 2003. 7:119–120.
3. Levine DP. Vancomycin: a history. Clin Infect Dis. 2006. 42:Suppl 1. S5–S12.
4. Kupstaite R, Baranauskaite A, Pileckyte M, Sveikata A, Kadusevicius E, Muckiene G. Severe vancomycin-induced anaphylactic reaction. Medicina (Kaunas). 2010. 46:30–33.
5. Rocha JL, Kondo W, Baptista MI, Da Cunha CA, Martins LT. Uncommon vancomycin-induced side effects. Braz J Infect Dis. 2002. 6:196–200.
6. Shah RA, Musthaq A, Khardori N. Vancomycin-induced thrombocytopenia in a 60-year-old man: a case report. J Med Case Reports. 2009. 3:7290.
7. Kwon HS, Chang YS, Jeong YY, Lee SM, Song WJ, Kim HB, Kim YK, Cho SH, Kim YY, Min KU. A case of hypersensitivity syndrome to both vancomycin and teicoplanin. J Korean Med Sci. 2006. 21:1108–1110.
8. Shin YS, Lee YW, Choi YH, Park B, Jee YK, Choi SK, Kim EG, Park JW, Hong CS. Spontaneous reporting of adverse drug events by Korean regional pharmacovigilance centers. Pharmacoepidemiol Drug Saf. 2009. 18:910–915.
9. Kohno S, Yamaguchi K, Aikawa N, Sumiyama Y, Odagiri S, Aoki N, Niki Y, Watanabe S, Furue M, Ito T, Croos-Dabrera R, Tack KJ. Linezolid versus vancomycin for the treatment of infections caused by methicillin-resistant Staphylococcus aureus in Japan. J Antimicrob Chemother. 2007. 60:1361–1369.
10. Wood MJ. Comparative safety of teicoplanin and vancomycin. J Chemother. 2000. 12:Suppl 5. 21–25.
11. Thong BY. Update on the management of antibiotic allergy. Allergy Asthma Immunol Res. 2010. 2:77–86.
12. Wallace MR, Mascola JR, Oldfield EC 3rd. Red man syndrome: incidence, etiology, and prophylaxis. J Infect Dis. 1991. 164:1180–1185.
13. Polk RE, Healy DP, Schwartz LB, Rock DT, Garson ML, Roller K. Vancomycin and the red-man syndrome: pharmacodynamics of histamine release. J Infect Dis. 1988. 157:502–507.
14. O'Sullivan TL, Ruffing MJ, Lamp KC, Warbasse LH, Rybak MJ. Prospective evaluation of red man syndrome in patients receiving vancomycin. J Infect Dis. 1993. 168:773–776.
15. Levy JH, Kettlekamp N, Goertz P, Hermens J, Hirshman CA. Histamine release by vancomycin: a mechanism for hypotension in man. Anesthesiology. 1987. 67:122–125.
16. Zuliani E, Zwahlen H, Gilliet F, Marone C. Vancomycin-induced hypersensitivity reaction with acute renal failure: resolution following cyclosporine treatment. Clin Nephrol. 2005. 64:155–158.
17. Tamagawa-Mineoka R, Katoh N, Nara T, Nishimura Y, Yamamoto S, Kishimoto S. DRESS syndrome caused by teicoplanin and vancomycin, associated with reactivation of human herpesvirus-6. Int J Dermatol. 2007. 46:654–655.
18. Yazganoglu KD, Ozkaya E, Ergin-Ozcan P, Cakar N. Vancomycin-induced drug hypersensitivity syndrome. J Eur Acad Dermatol Venereol. 2005. 19:648–650.
19. Vauthey L, Uçkay I, Abrassart S, Bernard L, Assal M, Ferry T, Djordjevic M, Roussos C, Vaudaux P. Vancomycin-induced DRESS syndrome in a female patient. Pharmacology. 2008. 82:138–141.
20. Polk RE, Israel D, Wang J, Venitz J, Miller J, Stotka J. Vancomycin skin tests and prediction of "red man syndrome" in healthy volunteers. Antimicrob Agents Chemother. 1993. 37:2139–2143.
21. Perrin-Lamarre A, Petitpain N, Trechot P, Cuny JF, Schmutz JL, Barbaud A. Glycopeptide-induced cutaneous adverse reaction: results of an immunoallergic investigation in eight patients. Ann Dermatol Venereol. 2010. 137:101–105.
22. Rosow CE, Moss J, Philbin DM, Savarese JJ. Histamine release during morphine and fentanyl anesthesia. Anesthesiology. 1982. 56:93–96.
23. Moss J, Rosow CE. Histamine release by narcotics and muscle relaxants in humans. Anesthesiology. 1983. 59:330–339.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download