Abstract
B cells are generally considered to positively regulate immune responses by producing antigen-specific antibodies. B cells are classified into classical CD5- conventional B cells and CD5+ B1 cells. The latter produce multi-specific autoantibodies and are thought to be involved in autoimmune diseases. However, evidence supporting a B cell negative regulatory function has accumulated over the past 30 years. Multiple reports have suggested that absence, or loss, of regulatory B cells exacerbates symptoms of both allergic (including contact hypersensitivity and anaphylaxis) and autoimmune (such as experimental autoimmune encephalomyelitis, chronic colitis, and collagen-induced arthritis) diseases, and in lupus-like models of autoimmunity. Regulatory B cells are characterized by production of the negative regulatory cytokines, IL-10 and TGF-β. IL-10-producing B cells were the first regulatory B cells to be recognized and were termed 'B10' cells. IL-10-producing regulatory B cells are of the CD19+CD5+IgMhiIgDloCD1dhi type. Recently, a TGF-β-producing regulatory B cell subset, Br3, has been shown to be related to immune tolerance in food allergies. Moreover, forkhead box P3 (Foxp3)-expressing B cells have also been identified in humans and may act as regulatory B cells (Bregs). The functional image of regulatory B cells is similar to that of regulatory T cells. Because of the proliferative and apoptotic responses of Br1 and Br3 cells in immune tolerance in non-IgE-mediated food allergy, reciprocal roles and counter-regulatory mechanisms of Br1 and Br3 responses are also suspected. Additionally, different roles for regulatory B and T cells at different time points during initiation and progression of autoimmune disease are described.
Studies of immune mechanisms have focused on the effector functions of immune responses to pathogens or the immunopathogenesis of autoimmune diseases. The discovery of CD4+Foxp3+ regulatory T cells (Treg), which suppress antigen (Ag)-specific immune effector cells, made negative immune regulation a focus of work to characterize the pathogenesis of autoimmunologic and allergic inflammatory diseases.
B cells that have ability to terminally differentiate into antibody (Ab)-secreting plasma cells, are generally considered to positively regulate immune responses by producing Ag-specific Abs and helping to induce optimal CD4+ T cell activation.1,2 B cells can also serve as antigen-presenting cells (APCs).3 B cell-specific expression of co-stimulatory molecules, such as CD80, CD86, and OX40L, is also important for optimal T cell activation.4,5
Evidence supporting B cell negative regulatory function has accumulated over the past 30 years. B cells and specific subsets thereof can negatively regulate immune responses in mice.6-10 The absence, or loss, of these regulatory B cells exacerbates disease symptoms in allergic (including contact hypersensitivity and anaphylaxis), and autoimmune (such as experimental autoimmune encephalomyelitis, chronic colitis, collagen induced arthritis) diseases, and in lupus-like models of autoimmunity.11-19 Moreover, B cells regulate immunological or allergic inflammation and T cell-mediated autoimmunity through the production of IL-10.12-14,16-20 With these background, recent advances in knowledge of regulatory B cells and their clinical and immunological significance in allergy are described in this review.
Ly-1/CD5 is a 68 kDa glycoprotein that was originally thought to mark the helper subset of T cells.21 It was shown to be present not only on all T cells, but also on a subset of B cells. In 1983, Hayakwawa et al.22 originally distinguished a subset of mouse B cells that bear the pan-T cell glycoprotein Ly-1 (CD5). Evidence of the existence of a CD5-bearing B cell subset in humans was reported in 1986.23 T cell markers were subsequently shown to be shared by B cells in some patients with chronic lymphocytic leukemia.24 CD5, the human counterpart of the Ly-1 molecule in the mouse, is detectable, but is weakly expressed only on a minute fraction of circulating B cells.25
Thereafter, CD5 expression served as a useful marker for a functionally distinct population of B cells that has attracted considerable interest from investigators of both the murine and human immune systems.21 B cells are classified according to CD5 expression as CD5+ B1 cells and CD5- conventional B2 cells. B1 cells emerge early in development, are abundant in the peritoneal and pleural cavities, and are defined by their surface marker expression pattern as B220lo, IgMhi, IgD+, CD9+, CD43+, and CD23lo. B2 cells are defined phenotypically as B220hi, IgMhi/lo, IgD+, CD9-, CD43-, and CD23hi. B1 cells are long-lived, self-renewing cells that produce polyreactive IgM, known as "natural antibodies," and do not undergo extensive somatic hypermutation. The human B1 cell repertoire includes two distinct subsets: B1a cells, which develop from progenitors in the fetal splanchnic district, namely the omentum, and are maintained in adult life by virtue of their self-replenishing nature, and B1b cells, progenitors of which can be found in the splanchnic district and, perhaps, adult bone marrow. B2 cells, which arise in the fetal liver and are continuously replenished in adult life by progenitors in the bone marrow, are capable of generating hypermutated antibodies, and play a central role in conventional adaptive humoral immune responses. Immature B2 cells develop in the bone marrow and migrate to the spleen, and are then called transitional B cells.26
B1 cells play a major role in autoimmunity and constitute the physiological equivalent of the neoplastic forms in various lymphoproliferative disorders, such as chronic lymphocytic leukemia (CLL), which are often associated with the production of monoclonal antibodies to self antigens. Human B1a (CD5+ B) and B1b (CD5- CD45RAlo B) cells are responsible for the production of natural (polyreactive and monoreactive) antibodies in the fetus, neonate, and adult, and can give rise to autoantibodies producing cells characteristic of several autoimmune disease states. However, non-classical B1 cell functions are critical in the manifestation of several autoimmune diseases. Independent of autoantibody production, B cells are essential for disease initiation or progression, either through APC function or the secretion of proinflammatory cytokines.27
Regulatory B cells, a "new" B cell population that suppresses immune responses, have been highlighted recently, in contrast to normal B cells, which function to augment immune responses. The term 'regulatory B cells' to designate B cells with inhibitory properties, was used for the first time by Mizoguchi and Bhan.13,28 B cell deficiency in T cell receptor (TCR) α-deficient mice, which spontaneously develop colitis, results in an earlier onset of disease and exacerbated intestinal inflammation. Also, a suppressive function for IL-10-producing B cells (Br1 or B10) in collagen-induced arthritis (CIA) was reported.14 Overall, these data suggest that regulatory B cells are key regulators of many disease states due to their production of IL-10.
Over the past decade, B cell negative regulation has been demonstrated in mouse autoimmunity models.8,16,29 A phenotypically unique (CD1dhiCD5+CD19hi) negative regulatory B cell subset in the spleens of naïve wild-type mice has been identified.17 This regulatory B cell subset is Ag-specific and significantly influences T cell activation and inflammatory responses through IL-10 production.17,30 The IL-10-producing CD1dhiCD5+CD19hi regulatory subset designated 'B10' by Tedder and colleagues is responsible for most IL-10 production.30
IL-10 is multi-potent in immune modulation, exerting anti-inflammatory and suppressive effects on most hematopoietic cells.9 IL-10 is involved in not only inhibition of Th1 polarization but also prevents Th2 responses. Pro-inflammatory cytokine production by monocytes and macrophages is suppressed by IL-10.31 IL-10 expression in the mucosal environment is also critical for the generation of immunological tolerance.32 Although IL-10 generally plays immunosuppressive roles, it also has pleiotropic and immunostimulatory activities. IL-10 released in local microenvironments is more critical for cell-to-cell interactions than systemic IL-10.
Many groups have identified IL-10-producing regulatory B cells, but only a few studies have attributed these characteristics to specific B cell subpopulations.9 IL-10-producing B10 cells are relatively rare within the spleen. Splenic IL-10+ cells expressed a CD21hiCD23+IgMhi CD1dhiCD93int phenotype, which is similar to the T2-marginal zone (MZ) precursor cell phenotype. The B10 cell phenotype is unique, but subsets thereof are phenotypically diverse. The categorization of B10 cells as CD1dhiCD5+ has been useful in defining the novel character of these B cells and for analyzing their distributions.16 CD1d is expressed on a wide variety of cell types, including constitutive expression by most B cells.33 In the mouse spleen, CD1d expression is higher on B cells of both the CD21hiIgMhiIgDloCD24intCD23-CD43- MZ and the CD21hiIgMhiIgDhiCD23+ T2 B cell subsets.33
Concerning CD19 expression by IL-10-producing regulatory B cells, although Br1 has been reported in the CD19hi phenotype group, most reports place it in the CD19lo subpopulations.34 Further studies are needed to explain this discrepancy.
Regulatory B cells have been characterized as having B1a, MZ, and T2-MZ precursor B cell origins. B10 cells are derived from B10 progenitor (B10pro) cells that may not fit into any preconceived subset.35 Neonatal mouse spleen B cells are IL-10-competent CD1dloCD5+ B10pro cells. Prolonged stimulation induces the CD1dhiCD5+ B cell subset to mature.9 Anti-CD40 mAb and LPS induce maturation of B10 and B10pro cells.
B10 cell regulation of inflammation and autoimmunity is Ag-specific.17,30 Furthermore, B10 cell development is significantly influenced by B cell Ag receptor (BCR), because B10 cell numbers are reduced by approximately 90% in transgenic mice with a fixed BCR.30 B10 cell frequency and numbers are also decreased by 70-80% in mice lacking CD19, which enhances transmembrane and BCR signaling and humoral immunity. CD40L dramatically enhanced the numbers of CD1dhiCD5+ B cells and IL-10-producing B cells.16 BCR diversity and CD19-generated signals are critical for normal B10 cell development and/or peripheral expansion, while enhanced B cell signaling significantly enhances B10 cell generation.
Despite the requirement for BCR expression during B10 cell development, B cell stimulation with mitogenic anti-IgM Ab does not induce cytoplasmic IL-10 expression.30 BCR-generated signals inhibit the abilities of LPS and anti-CD40 mAb to induce cytoplasmic IL-10 production. B10 development appears to be T cell-independent. Major histocompatibility complex (MHC) class I and class II molecules and CD1d expression are not required for B10 cell development.30
B10 and B10pro cells, which secrete IL-10 after stimulation with CD40 agonistic antibody for 48 hours), are found in many tissues. B10 and B10pro cells have been identified in blood, bone marrow, lymph node, spleen, and the peritoneal cavity of adult mice.9,16,17,30 B10 and B10pro cells normally represent about 1-2 and 7-9% of adult mouse splenic B cells, respectively. B10 cells (2-3% of CD19+ B cells) and B10pro cells (5-6% of CD19+ B cells) are also present in adult mouse bone marrow. The peritoneal cavity may be a major B10 cell source, with up to 10% of B cells producing IL-10. Strikingly, 30-35% of peritoneal cavity B cells are B10pro cells, while peripheral blood and lymph nodes contain few, if any, B10 cells but 3-8% of the total B cell population are B10pro cells.9,17 Thus, B10 cells may be located predominantly within the spleen and peritoneal cavity.
CD1dhiCD5+ B cells are responsible for most IL-10 production by B cells.16 IL-10-producing B cells have been regarded as regulatory B cells and this specific subset of regulatory B cells has been termed "B10 cells." Another regulatory B cell subset that produces transforming growth factor-β (TGF-β) after stimulation with LPS has been also identified.36 TGF-β is produced by both CD5+ and CD5- B cells from autoimmune-prone NZB mice,37 and is also expressed by normal human B cells.38
TGF-β-producing B cells are regarded as another regulatory type. These cells play essential roles in the induction of tolerance to non-IgE mediated food allergy in atopic dermatitis.38 In a recent study, Foxp3-expressing B cells were identified within CD19+CD5+ B cells and were proposed to be regulatory B cells (Breg), comparable to Treg.39
T and B cells are functionally classified into effector and negative regulatory cells. The latter type that produce negative regulatory/suppressive cytokines, such as TGF-β and IL-10, or express the transcription factor, Foxp3, are categorized as regulatory T and B cells (Fig. 1). The components of the regulatory B cell system are similar to those of regulatory T cells, including Type I IL-10- producing regulatory T cells (Tr1), Type III TGF-β-producing helper T cells (Th3), and Treg. TGF-β induces apoptosis in CD5+ B cells,37 and IL-10 enhances the proliferation of CD5+ cells in an autocrine manner.40 Reciprocal effects of IL-10 from Br1 and TGF-β from Br3 are shown in Fig. 2. Also, IL-10 from Tr1 cells and TGF-β from Th3 cells have the same effect on regulatory B cells.
Given recent advances in our understanding of the complexity of regulatory B cells, a systemic nomenclature is necessary. Lee and Noh named regulatory B cells systematically. First, considering the classification and nomenclature of CD5+ B1 and CD5- B2 cells, B10 cells may be another subset in addition to B1 and B2 cells. The composition of regulatory B cells is similar to that of regulatory T cells. IL-10-producing regulatory B cells are termed 'Br1,' similar to Type I IL-10-producing regulatory 'Tr1' T cells. The nomenclature of Br1 cells (i.e., IL-10-producing regulatory B cells) should be made more reasonable.41 Second, TGF-β-producing B cells are termed 'Br3,' like 'Th3' T cells. Third, recently, CD19+CD5+Foxp3+ B cells, Foxp3-expressing regulatory B cells, are designated Breg, similar to 'Treg,' CD4+Foxp3+ T cells.39 Finally, the functional structure of regulatory B cells may be similar to that of regulatory T cells.
Regulatory B cells are more highly apoptotic than T cells in human food allergies and atopic dermatitis. Br1 cells are more highly apoptotic than Tr1 cells41; furthermore, Br338 and Breg cells39 are more apoptotic than either Th3 or Treg cells.
Recently, counter-regulation of immune responses has been suggested as a mechanism that prevents excessive immune reactions, and the apoptotic activity of regulatory B cells may be one counter-regulatory mechanism.38,39,41 The physiological characteristics of regulatory B cells, including those relating to apoptosis, differ from those of regulatory T cells.
Th2.52 cells (i.e., CD5+, B cell lines) are used for the study of the effects of cytokines on apoptosis of regulatory B cells. IFN-γ-induced marked NO production through expression of the inducible type of NO synthase (iNOS) and cell death.42 IFN-γ-induced NO production and cell death were inhibited by IL-4.43 Chronic lymphocytic leukemia (CLL) is a leukemic disease of CD5+ B cells. IL-10 enhanced the survival of B-CLL cells in a dose-dependent manner by inhibiting apoptotic cell death,40 and acts as an autocrine growth factor for B-CLL cells. Recombinant IL-6 is also important for growth,44 and protect CLL cells from apoptosis. TGF-β negatively regulates chronic lymphocytic leukemia,45 and exhibits an antiproliferative effect on B-CLL cells in an autocrine manner.46 TGF-β not only stimulates B cell apoptosis, but also induces apoptosis in CD5+ B cells.35
Contact hypersensitivity is considered a prototypic Th1 cell-mediated inflammatory reaction in which B cells are not involved in antigen presentation and, therefore, have no or at most a very limited role in augmenting inflammation. The contact hypersensitivity response is exacerbated in CD19-deficient mice.47 CD19 expression is also critical in the regulatory B cell subset, and CD19 loss results in a defect in regulatory B cells. The splenic B cell subset which produces IL-10 displays a CD1dhiCD5+phenotype. IL-10-deficient mice also possess CD1dhiCD5+ B cells, although adoptive transfer of IL-10-deficient CD1dhiCD5+ B cells does not ameliorate contact hypersensitivity responses, suggesting that this suppression is IL-10-dependent.
B1 cells in the peritoneal cavity are a rich source of IL-10, and suppress contact hypersensitivity, particularly in the late phase.48 CD22-deficient mice showed delayed recovery from contact hypersensitivity reactions compared with wild-type mice. Adoptive transfer of wild-type peritoneal B1 cells, but not IL-10-deficient peritoneal B1 cells, reverses the prolonged hypersensitivity reactions of CD22- and CD19-deficient mice. Simultaneous injection of anti-IL-10 receptor antibody inhibits these phenomena. Two distinct regulatory B cell subsets cooperatively inhibit contact hypersensitivity responses. Splenic CD1dhiCD5+ B cells have a crucial role in suppressing the acute exacerbating phase of contact hypersensitivity, while peritoneal B1a cells likely suppress the late remission phase, although their role appears less significant than that of splenic B10 cells. The role of B1 cells in contact hypersensitivity remains controversial. Tsuji et al.49 reported a promoting, rather than inhibitory, role for peritoneal B1 cells in contact hypersensitivity. While B1 cells do indeed play a protective role in contact hypersensitivity,50 it is possible that peritoneal B1 cells have both promoting and suppressive roles.
Allergic asthma is a chronic inflammatory disease of the airways associated with airway hyper responsiveness (AHR) to inhaled allergens and dysregulated type 2 immunity.51 Dysregulated type 2 immunity in allergic asthma is characterized by an expansion of CD4+ Th-cell populations, with increased production of Th2 cytokines (IL-4, IL-5, IL-13), elevated total and allergen-specific IgE, eosinophilia, and airway inflammation. IL-10-producing B cells have been shown to downregulate inflammation in airway hyper-responsiveness.48 Also, regulatory B cells were reported to be involved in the pathogenesis of delayed-type hypersensitivity via mediation of both early T cell recruitment52 and airway hyper-reactivity in non-atopic asthma.53 In another report, regulatory B cells were protective against allergic airway inflammation.54
Despite the fact that helminth-infected individuals display the characteristic type 2 responses seen in patients with allergy, helminths have evolved multiple mechanisms to regulate inflammatory responses in the infected host.55-58 Interest has focused on helminth induction of CD4+ T cells that produce the regulatory cytokines TGF-β and IL-10. Treg cells, including thymus-selected naturally occurring CD4+CD25+FoxP3+ Treg cells and inducible IL-10-secreting Treg type 1 cells (Tr1), are involved in modulation of allergic asthma.59 Infection with Schistosoma mansoni worms plays a role in suppression of allergen-induced AHR experimental murine models of allergic disease.48 S. mansoni-mediated protection from allergic airway inflammation was dependent on induction of both IL-10 and B cells.
IL-10-producing B cells suppress allergic inflammation.60 CD19+IL-10+CD1dhiCD5+CD21hiCD23+ IgD+ IgMhi Breg cells reverse allergic airway inflammation. Breg cell function is mediated by IL-10 and dependent on the expression of CD1d. Breg cells suppress airway inflammation by inducing the recruitment of natural Treg (CD4+CD25+FoxP3+) cells to the lungs in a TGF-β-independent manner, with these Treg cells mediating the suppression of lung inflammation.
A major role for IL-10-producing B cells has been described in the downregulation of inflammation in anaphylaxis.60 S. mansoni worms increased numbers of IL-10-producing B cells, and transfer of these cells protected recipient mice against experimentally-induced anaphylaxis. Infection with S. mansoni worms plays a role in suppression of anaphylaxis and transfer of these cells protected recipient mice against experimentally-induced anaphylaxis. S. mansoni-mediated protection from anaphylaxis was dependent on induction of both IL-10 and B cells.
The clinical significance of regulatory B cells, including Br1 and Br3, in non-IgE mediated food allergy and atopic dermatitis has been described. Atopic dermatitis is a chronic relapsing allergic dermatitis and food allergy plays an important role in atopic dermatitis.61 An imbalance in the Th1/Th2 ratio is known to be important in the development of atopic dermatitis.62 Food allergies are classified as IgE-mediated, non-IgE-mediated, or mixed-type.63 Late eczematous reactions of the skin have been found to be diagnostic of non-IgE-mediated food allergy and almost identical to those in atopic dermatitis.64 Due to a lack of available laboratory diagnostic tests, non-IgE-mediated food allergy has been underdiagnosed and undertreated.65
Recently, the clinical significance of regulatory B cells in non-IgE-mediated food allergy has been investigated. Br1 cells proliferate in response to casein stimulation in milk-tolerant subjects,41 but not in milk allergy patients. Br3 cells also showed similar responses in non-IgE-mediated food allergy and proliferated in response to allergen stimulation in milk-tolerant subjects (Fig. 3).38 Br1 and Br3 cells are critical for induction of immune tolerance in non-IgE-mediated food allergy related to atopic dermatitis, while Treg responses have not been reported to be decisive in the development of either allergy or immune tolerance.
Most studies concerning human B-cell production of IL-10 have provided contradictory data, making the identification of regulatory B cells problematic. Human tonsil B cells secrete only low levels of IL-10 following BCR or CD40 ligation. While addition of exogenous IL-4 to cultures diminishes CD40-induced secretion of IL-10, addition of the Staphylococcus aureus Cowan I (SAC) polyclonal mitogen significantly increases IL-10 secretion.9 Epstein-Barr virus (EBV) infection of purified tonsil B cells induces high levels of IL-10. Neutralization of endogenous IL-10 does not alter the growth of CD40-activated B cells, but does inhibit their IgM, IgG, and IgA secretion. IL-10 may also synergize with IL-6 to sustain differentiation of CD40-activated B cells.
In contrast to mice, the main source of IL-10 in humans may not be CD5+ B cells. CD5+ B cells represent 40-60% of B cells in the human fetal spleen.66 CD5+ B cells are also present at high percentages in cord blood (~7%), while <2% of these cells exhibit cytoplasmic IL-10 staining. However, after 24 hours stimulation with PMA, 23% of cord blood CD5+ B cells produced IL-10.67 Nonetheless, human cord blood CD5+ B cells secrete only low levels of IL-10 following BCR or CD40 ligation.68
Blair et al.69 demonstrated that human CD19+CD24hiCD38hi B cells exhibit regulatory activity. After CD40 stimulation, CD19+CD24hiCD38hi B cells suppressed the differentiation of Th1 cells, partially via the provision of IL-10, but not TGF-β, and their suppressive capacity was reversed by addition of CD80 and CD86 mAb. CD19+CD24hiCD38hi B cells isolated from the peripheral blood of systemic lupus erythematosus (SLE) patients were refractory to CD40 stimulation, produced less IL-10, and lacked the suppressive capacity of their healthy counterparts. In contrast to murine regulatory B cells, the suppressive activity of human regulatory B cells is only partially dependent on IL-10. Regulatory B cells also exist in humans; however, their surface phenotype remains controversial.
Respiratory exposure to allergen induces development of allergen-specific CD4+ T cell tolerance that effectively protects against development of allergic-sensitization and T(h)2-biased immunity.70 CD4+Foxp3+CD25+ regulatory T cells play a dominant role in immune tolerance and modulate immune reactions by secreting immunomodulatory cytokines, particularly TGF-β.71 Similarly, TGF β-producing Br3, and TGF-β-producing Th3, cells seem to be involved in immune tolerance, including allergy tolerance.38 The presence of Foxp3+ regulatory B cells (Bregs) has recently been reported39 and are expected to have a negative regulatory role.
IL-10 produced by Br1 is involved in the autocrine growth of CD5+ B1 cells, including Br1 and Br3,40 while TGF-β, produced by Br3 cells, induces apoptosis of CD5+ B cells.38 During a tolerant reaction to allergen in non-IgE-mediated food allergy related to atopic dermatitis, Br1 and Br3 proliferate in response to allergen stimulation.38,40 Interestingly, Br1 and Br3 cells are not only highly apoptotic, compared with regulatory IL-10 (Tr1) and TGF-β (Th3)-producing T cells, but also show increased proliferation simultaneously with increasing apoptotic Br1 and Br3 cells in response to casein stimulation in milk-tolerant subjects. The proliferatory and counter-regulatory roles of IL-10 and TGF-β, respectively, may indicate the immunologic mechanisms of the effects of Br1 and Br3 cells in tolerance reactions (Fig. 4). Further functional studies may be needed to elucidate precisely the role of Bregs.
Recently, studies have focused on counter-regulation of excessive immune responses. Several mechanisms have been suggested to mediate prevention of excessive suppression of tolerant responses to allergen by Br1 and Br3 cells in non-IgE-mediated food allergy related to atopic dermatitis. 1) TGF-β produced by Br3 cells, which proliferate simultaneously with Br1 upon allergen stimulation, can induce apoptosis of regulatory B cells, including Br1 and Br3 cells. 2) Activation-induced apoptosis of B cells may also be an important negative regulatory mechanism.72 3) Recently, IL-10 produced by Br1 cells was suggested as a counter-regulatory mechanism through the IL-10/IL-27/IL-21 pathway.73 However, proliferation with activation and apoptosis seem to conflict. Indeed, the IL-21 pathway induces proliferation of B cells simultaneously with apoptosis.74 4) Br1-induced Treg19 suppress Br1, as described below.75 Thus, Tregs may act as a counter-regulatory mechanism for Br1 responses.
TGF-β, produced by either Th3 or Br3 cells, is critical for the function of CD4+CD25+Foxp3+ regulatory T cells.74 These cells play a dominant role in the maintenance of immune tolerance, and also modulate immune reactions by secreting immunomodulatory cytokines, particularly TGF-β.71,74 IL-10, produced by Tr1 and Br1 cells, drives the generation of Tr1 cells, which act to suppress antigen-specific immune responses and actively down-regulate pathological immune responses.76 IL-10 produced by Br1 cells may induce Tr1 cells, and also acts as an autocrine growth factor for CD5+ B cells, including Br1 and Br3 cells. Treg cells suppress B1 cells,75 while Br1 cells induce Treg cells in CD192/2 NZB/W mice.19 TGF-β, produced by Br3, Th3 and Treg cells, plays an important role in the development and function of CD4+CD25+Foxp3+ regulatory T cells.74 However, TGF-β induces apoptosis of B cells, including Br1 and Br3 cells. Thus, the reciprocal activities of Br1, Br3, Tr1, Th3, and Treg cells are known (Fig. 5) and their immunological roles should be investigated further.
Br1 cells have different regulatory functions than Tregs, because they function at different time points during the initiation and progression of autoimmune diseases.75 Br1 cells expand rapidly during experimental autoimmune encephalomyelitis (EAE) initiation and quickly inhibit disease severity, while Denileukin diftitox-induced Treg depletion had no effect on disease initiation, but exacerbated late-phase disease. Br1 cells predominantly reduced disease severity during EAE initiation through production of IL-10, whereas Tregs reciprocally inhibited late stage EAE immunopathogenesis. In this context, Br1 may be involved in the initiation of pathological responses and Tregs in their maintenance or progression.
Non-IgE-mediated food allergy is caused by allergen-specific Th2 cellular immune reactions. Specifically, induction of allergen tolerance involves cell-mediated responses, including Br1 and/or Br3 cells.38,40 The roles of Br1 and Treg in non-IgE mediated food allergy are expected to be similar to those in autoimmune disease (Fig. 6). Br1 cells play a role in early, and Tregs in later, phases of persistent allergic reactions. Autoantigen is continuously present, while the immune system is exposed to food antigen only intermittently and so prolonged persistent clinical allergic reactions are rare. Thus, Br1, rather than Treg, responses may be expected in food allergies. As expected, there has been no report of this change in the role of Tregs in food allergy despite knowledge of Br1 and Br3 responses.
Recently, there have been significant advances in understanding the development, regulation, and roles of IL-10-producing regulatory B cells (Br1 or B10) as well as other regulatory B cell subsets, including TGF-β-producing Br3 and Foxp3-expressing Bregs. While most studies have focused primarily on autoimmune disease, regulatory B cells are also clinically and immunologically significant in allergic diseases. Although there has been intensive investigation of Br1 cells, TGF-β-producing regulatory B cells (Br3) may also be involved in immune tolerance of non-IgE mediated food allergy related to atopic dermatitis. Currently, cytokine and cellular networks are investigated through assessing (i) their effects on regulatory B cells, (ii) reciprocal effects between regulatory B and T cells, and (iii) the proper role of regulatory T and B cells. Moreover, Foxp3+ regulatory B cells (Breg) appear to be compatible with Foxp3+ regulatory T cells (Treg). The functional structure of regulatory B cells is similar to that of regulatory T cells. Additionally, both regulatory T and B cells seem to negatively regulate allergic diseases including contact dermatitis, asthma, anaphylaxis and non-IgE-mediated food allergy related to atopic dermatitis. Finally, new regulatory B cell types are being discovered and so a systemic nomenclature is increasingly necessary.
Figures and Tables
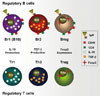 | Fig. 1Regulatory B and T cells. Tr1, Th3, and regulatory T (Treg) cells produce IL-10 and/or TGF-β, and express Foxp3. B cells also produce IL-10, TGF-β, and Foxp3; these are termed Br1, Br3, and regulatory B cells (Breg). The B cell system thus seems to be a mirror image of the T cell system. |
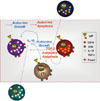 | Fig. 2Reciprocal roles of IL-10 from Br1 and TGF-β from Br3 cells on Br1 and Br3 cells. Br1 and Br3 cells belong to the CD5+ B cell subset. Growth of CD5+ B cells is stimulated by IL-10 in an autocrine manner, while TGF-β induces their apoptosis. IL-10 produced by Tr1 cells and TGF-β by Th3 cells have the same effects on CD5+ B cells. |
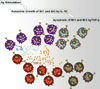 | Fig. 3Proliferative and simultaneous apoptotic responses of Br1 and Br3 cells to casein in milk-tolerant subjects. IL-10-producing Br1 and TGF-β-producing Br3 cells proliferate while simultaneously showing apoptotic changes. IL-10 may stimulate proliferation of Br1 and Br3 cells, while TGF-β may induce their apoptosis. |
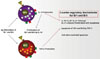 | Fig. 4Reconstruction of Br1 and Br3 cell counter-regulatory mechanisms during induction of tolerance to allergen stimulation. IL-10 produced by Br1 cells mediates counter-regulatory effects through the IL-10/IL-27/IL-21 pathway. TGF-β produced by Br3 cells also induced apoptosis of both Br1 and Br3 cells. Moreover, activation-mediated apoptosis plays a role in counter-regulation of immune responses. |
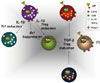 | Fig. 5Reciprocal roles of regulatory B and T cells. IL-10 from Br1 and Tr1 cells induces Tr1 and Treg cells. TGF-β produced by Br3 and Th3 cells induces Treg cells, which then act to suppress Br1 cells. |
 | Fig. 6Regulatory roles of regulatory B and T cells. Regulatory T and B cells function at different time points during initiation and progression of autoimmune diseases. Br1 and Br3 cells are decisive for tolerance induction in non-IgE-mediated food allergy related to atopic dermatitis. In contrast, there has been no report of the role of Tregs in the development of allergy or tolerance. The roles of regulatory T and B cells in non-IgE-mediated food allergy related to atopic dermatitis may thus be similar to those in autoimmune disease. |
ACKNOWLEDGMENTS
I express my special appreciation to Sunheui Cho (Department of Food & Nutrition, College of Human Ecology, Hanyang University, Seoul, Korea) who produced the medical illustrations in this review.
References
1. LeBien TW, Tedder TF. B lymphocytes: how they develop and function. Blood. 2008. 112:1570–1580.
2. DiLillo DJ, Hamaguchi Y, Ueda Y, Yang K, Uchida J, Haas KM, Kelsoe G, Tedder TF. Maintenance of long-lived plasma cells and serological memory despite mature and memory B cell depletion during CD20 immunotherapy in mice. J Immunol. 2008. 180:361–371.
3. Liu Y, Wu Y, Ramarathinam L, Guo Y, Huszar D, Trounstine M, Zhao M. Gene-targeted B-deficient mice reveal a critical role for B cells in the CD4 T cell response. Int Immunol. 1995. 7:1353–1362.
4. O'Neill SK, Cao Y, Hamel KM, Doodes PD, Hutas G, Finnegan A. Expression of CD80/86 on B cells is essential for autoreactive T cell activation and the development of arthritis. J Immunol. 2007. 179:5109–5116.
5. Linton PJ, Bautista B, Biederman E, Bradley ES, Harbertson J, Kondrack RM, Padrick RC, Bradley LM. Costimulation via OX40L expressed by B cells is sufficient to determine the extent of primary CD4 cell expansion and Th2 cytokine secretion in vivo. J Exp Med. 2003. 197:875–883.
6. Fillatreau S, Gray D, Anderton SM. Not always the bad guys: B cells as regulators of autoimmune pathology. Nat Rev Immunol. 2008. 8:391–397.
7. Rieger A, Bar-Or A. B-cell-derived interleukin-10 in autoimmune disease: regulating the regulators. Nat Rev Immunol. 2008. 8:486–487.
8. Mauri C, Ehrenstein MR. The 'short' history of regulatory B cells. Trends Immunol. 2008. 29:34–40.
9. DiLillo DJ, Matsushita T, Tedder TF. B10 cells and regulatory B cells balance immune responses during inflammation, autoimmunity, and cancer. Ann N Y Acad Sci. 2010. 1183:38–57.
10. Lund FE, Randall TD. Effector and regulatory B cells: modulators of CD4+ T cell immunity. Nat Rev Immunol. 2010. 10:236–247.
11. Mizoguchi A, Mizoguchi E, Smith RN, Preffer FI, Bhan AK. Suppressive role of B cells in chronic colitis of T cell receptor alpha mutant mice. J Exp Med. 1997. 186:1749–1756.
12. Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM. B cells regulate autoimmunity by provision of IL-10. Nat Immunol. 2002. 3:944–950.
13. Mizoguchi A, Mizoguchi E, Takedatsu H, Blumberg RS, Bhan AK. Chronic intestinal inflammatory condition generates IL-10-producing regulatory B cell subset characterized by CD1d upregulation. Immunity. 2002. 16:219–230.
14. Mauri C, Gray D, Mushtaq N, Londei M. Prevention of arthritis by interleukin 10-producing B cells. J Exp Med. 2003. 197:489–501.
15. Evans JG, Chavez-Rueda KA, Eddaoudi A, Meyer-Bahlburg A, Rawlings DJ, Ehrenstein MR, Mauri C. Novel suppressive function of transitional 2 B cells in experimental arthritis. J Immunol. 2007. 178:7868–7878.
16. Yanaba K, Bouaziz JD, Haas KM, Poe JC, Fujimoto M, Tedder TF. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity. 2008. 28:639–650.
17. Matsushita T, Yanaba K, Bouaziz JD, Fujimoto M, Tedder TF. Regulatory B cells inhibit EAE initiation in mice while other B cells promote disease progression. J Clin Invest. 2008. 118:3420–3430.
18. Haas KM, Watanabe R, Matsushita T, Nakashima H, Ishiura N, Okochi H, Fujimoto M, Tedder TF. Protective and pathogenic roles for B cells during systemic autoimmunity in NZB/W F1 mice. J Immunol. 2010. 184:4789–4800.
19. Watanabe R, Ishiura N, Nakashima H, Kuwano Y, Okochi H, Tamaki K, Sato S, Tedder TF, Fujimoto M. Regulatory B cells (B10 cells) have a suppressive role in murine lupus: CD19 and B10 cell deficiency exacerbates systemic autoimmunity. J Immunol. 2010. 184:4801–4809.
20. Amu S, Saunders SP, Kronenberg M, Mangan NE, Atzberger A, Fallon PG. Regulatory B cells prevent and reverse allergic airway inflammation via FoxP3-positive T regulatory cells in a murine model. J Allergy Clin Immunol. 2010. 125:1114–1124.
21. Hardy RR. Isolation of Ly-1+/CD5+ B cells by cell sorting. Curr Protoc Immunol. 2003. Chapter 3:Unit 3 5B.
22. Hayakawa K, Hardy RR, Parks DR, Herzenberg LA. The "Ly-1 B" cell subpopulation in normal immunodefective, and autoimmune mice. J Exp Med. 1983. 157:202–218.
23. Gadol N, Ault KA. Phenotypic and functional characterization of human Leu1 (CD5) B cells. Immunol Rev. 1986. 93:23–34.
24. Schroeder HW Jr, Dighiero G. The pathogenesis of chronic lymphocytic leukemia: analysis of the antibody repertoire. Immunol Today. 1994. 15:288–294.
25. Youinou P, Mackenzie L, Broker BM, Isenberg DI, Drogou-Lelong A, Gentric A, Lydyard PM. The importance of CD5-positive B cells in nonorgan-specific autoimmune diseases. Scand J Rheumatol Suppl. 1988. 76:243–249.
26. Kasaian MT, Casali P. Autoimmunity-prone B-1 (CD5 B) cells, natural antibodies and self recognition. Autoimmunity. 1993. 15:315–329.
27. Shlomchik MJ, Craft JE, Mamula MJ. From T to B and back again: positive feedback in systemic autoimmune disease. Nat Rev Immunol. 2001. 1:147–153.
28. Mizoguchi A, Bhan AK. A case for regulatory B cells. J Immunol. 2006. 176:705–710.
29. Bouaziz JD, Yanaba K, Tedder TF. Regulatory B cells as inhibitors of immune responses and inflammation. Immunol Rev. 2008. 224:201–214.
30. Yanaba K, Bouaziz JD, Matsushita T, Tsubata T, Tedder TF. The development and function of regulatory B cells expressing IL-10 (B10 cells) requires antigen receptor diversity and TLR signals. J Immunol. 2009. 182:7459–7472.
31. Roncarolo MG, Gregori S, Battaglia M, Bacchetta R, Fleischhauer K, Levings MK. Interleukin-10-secreting type 1 regulatory T cells in rodents and humans. Immunol Rev. 2006. 212:28–50.
32. Berg DJ, Davidson N, Kühn R, Müller W, Menon S, Holland G, Thompson-Snipes L, Leach MW, Rennick D. Enterocolitis and colon cancer in interleukin-10-deficient mice are associated with aberrant cytokine production and CD4+ TH1-like responses. J Clin Invest. 1996. 98:1010–1020.
33. Amano M, Baumgarth N, Dick MD, Brossay L, Kronenberg M, Herzenberg LA, Strober S. CD1 expression defines subsets of follicular and marginal zone B cells in the spleen: β2-microglobulin-dependent and independent forms. J Immunol. 1998. 161:1710–1717.
34. Lee JH, Noh J, Noh G, Choi WS, Cho S, Lee SS. IL-10 is predominantly produced by the CD19(low)CD5(+) regulatory B cell subpopulation: characterisation of CD19(high) and CD19(low) subpopulations of CD5(+) B cells. Yonsei Med J. 2011. 09. Forthcoming.
35. Vitale G, Mion F, Pucillo C. Regulatory B cells: evidence, developmental origin and population diversity. Mol Immunol. 2010. 48:1–8.
36. Lotz M, Ranheim E, Kipps TJ. Transforming growth factor b as endogenous growth inhibitor of chronic lymphocytic leukemia B cells. J Exp Med. 1994. 179:999–1004.
37. Douglas RS, Woo EY, Capocasale RJ, Tarshis AD, Nowell PC, Moore JS. Altered response to and production of TGF-β by B cells from autoimmune NZB mice. Cell Immunol. 1997. 179:126–137.
38. Lee JH, Noh J, Noh G, Choi WS, Cho S, Lee SS. Allergen-specific transforming growth factor-β-producing CD19(+)CD5(+) regulatory B-cell (Br3) responses in human late eczematous allergic reactions to cow's milk. J Interferon Cytokine Res. 2011. 31:441–449.
39. Noh J, Choi WS, Noh G, Lee JH. Presence of Foxp3-expressing CD19(+)CD5(+) B cells in human peripheral blood mononuclear cells: human CD19(+)CD5(+)Foxp3(+) regulatory B cell (Breg). Immune Netw. 2010. 10:247–249.
40. Kitabayashi A, Hirokawa M, Miura AB. The role of interleukin-10 (IL-10) in chronic B-lymphocytic leukemia: IL-10 prevents leukemic cells from apoptotic cell death. Int J Hematol. 1995. 62:99–106.
41. Noh J, Lee JH, Noh G, Bang SY, Kim HS, Choi WS, Cho S, Lee SS. Characterisation of allergen-specific responses of IL-10-producing regulatory B cells (Br1) in cow milk allergy. Cell Immunol. 2010. 264:143–149.
42. Koide N, Sugiyama T, Mu MM, Mori I, Yoshida T, Hamano T, Yokochi T. Gamma interferon-induced nitric oxide production in mouse CD5+ B1-like cell line and its association with apoptotic cell death. Microbiol Immunol. 2003. 47:669–679.
43. Panayiotidis P, Ganeshaguru K, Jabbar SA, Hoffbrand AV. Interleukin-4 inhibits apoptotic cell death and loss of the bcl-2 protein in B-chronic lymphocytic leukaemia cells in vitro. Br J Haematol. 1993. 85:439–445.
44. Reittie JE, Yong KL, Panayiotidis P, Hoffbrand AV. Interleukin-6 inhibits apoptosis and tumour necrosis factor induced proliferation of B-chronic lymphocytic leukaemia. Leuk Lymphoma. 1996. 22:83–90.
45. Lagneaux L, Delforge A, Bron D, Massy M, Bernier M, Stryckmans P. Heterogenous response of B lymphocytes to transforming growth factor-β in B-cell chronic lymphocytic leukaemia: correlation with the expression of TGF-β receptors. Br J Haematol. 1997. 97:612–620.
46. Schuler M, Tretter T, Schneller F, Huber C, Peschel C. Autocrine transforming growth factor-β from chronic lymphocytic leukemia-B cells interferes with proliferative T cell signals. Immunobiology. 1999. 200:128–139.
47. Watanabe R, Fujimoto M, Ishiura N, Kuwano Y, Nakashima H, Yazawa N, Okochi H, Sato S, Tedder TF, Tamaki K. CD19 expression in B cells is important for suppression of contact hypersensitivity. Am J Pathol. 2007. 171:560–570.
48. Mangan NE, van Rooijen N, McKenzie AN, Fallon PG. Helminth-modified pulmonary immune response protects mice from allergen-induced airway hyperresponsiveness. J Immunol. 2006. 176:138–147.
49. Tsuji RF, Szczepanik M, Kawikova I, Paliwal V, Campos RA, Itakura A, Akahira-Azuma M, Baumgarth N, Herzenberg LA, Askenase PW. B cell-dependent T cell responses: IgM antibodies are required to elicit contact sensitivity. J Exp Med. 2002. 196:1277–1290.
50. Nakashima H, Hamaguchi Y, Watanabe R, Ishiura N, Kuwano Y, Okochi H, Takahashi Y, Tamaki K, Sato S, Tedder TF, Fujimoto M. CD22 expression mediates the regulatory functions of peritoneal B-1a cells during the remission phase of contact hypersensitivity reactions. J Immunol. 2010. 184:4637–4645.
51. Matsumura Y, Byrne SN, Nghiem DX, Miyahara Y, Ullrich SE. A role for inflammatory mediators in the induction of immunoregulatory B cells. J Immunol. 2006. 177:4810–4817.
52. Szczepanik M, Akahira-Azuma M, Bryniarski K, Tsuji RF, Kawikova I, Ptak W, Kiener C, Campos RA, Askenase PW. B-1 B cells mediate required early T cell recruitment to elicit protein-induced delayed-type hypersensitivity. J Immunol. 2003. 171:6225–6235.
53. Kawikova I, Paliwal V, Szczepanik M, Itakura A, Fukui M, Campos RA, Geba GP, Homer RJ, Iliopoulou BP, Pober JS, Tsuji RF, Askenase PW. Airway hyper-reactivity mediated by B-1 cell immunoglobulin M antibody generating complement C5a at 1 day post-immunization in a murine hapten model of non-atopic asthma. Immunology. 2004. 113:234–245.
54. Lundy SK, Berlin AA, Martens TF, Lukacs NW. Deficiency of regulatory B cells increases allergic airway inflammation. Inflamm Res. 2005. 54:514–521.
55. Yazdanbakhsh M, Kremsner PG, van Ree R. Allergy, parasites, and the hygiene hypothesis. Science. 2002. 296:490–494.
56. Fallon PG, Mangan NE. Suppression of TH2-type allergic reactions by helminth infection. Nat Rev Immunol. 2007. 7:220–230.
57. Anthony RM, Rutitzky LI, Urban JF Jr, Stadecker MJ, Gause WC. Protective immune mechanisms in helminth infection. Nat Rev Immunol. 2007. 7:975–987.
58. Maizels RM, Balic A, Gomez-Escobar N, Nair M, Taylor MD, Allen JE. Helminth parasites--masters of regulation. Immunol Rev. 2004. 201:89–116.
59. Hawrylowicz CM, O'Garra A. Potential role of interleukin-10-secreting regulatory T cells in allergy and asthma. Nat Rev Immunol. 2005. 5:271–283.
60. Mangan NE, Fallon RE, Smith P, van Rooijen N, McKenzie AN, Fallon PG. Helminth infection protects mice from anaphylaxis via IL-10-producing B cells. J Immunol. 2004. 173:6346–6356.
61. Sampson HA. Role of immediate food hypersensitivity in the pathogenesis of atopic dermatitis. J Allergy Clin Immunol. 1983. 71:473–480.
62. Grewe M, Bruijnzeel-Koomen CA, Schopf E, Thepen T, Langeveld-Wildschut AG, Ruzicka T, Krutmann J. A role for Th1 and Th2 cells in the immunopathogenesis of atopic dermatitis. Immunol Today. 1998. 19:359–361.
63. Ramesh S. Food allergy overview in children. Clin Rev Allergy Immunol. 2008. 34:217–230.
64. Kemp AS. Egg allergy. Pediatr Allergy Immunol. 2007. 18:696–702.
65. Sabra A, Bellanti JA, Rais JM, Castro HJ, de Inocencio JM, Sabra S. IgE and non-IgE food allergy. Ann Allergy Asthma Immunol. 2003. 90:71–76.
66. Antin JH, Emerson SG, Martin P, Gadol N, Ault KA. Leu-1+ (CD5+) B cells. A major lymphoid subpopulation in human fetal spleen: phenotypic and functional studies. J Immunol. 1986. 136:505–510.
67. Villaseñor-Bustamante S, Alvarado-De La Barrera C, Richaud-Patin Y, Martinez-Ayala H, Llorente L. Possible role of interleukin-10 in autoantibody production and in the fate of human cord blood CD5+ B lymphocytes. Scand J Immunol. 1999. 49:629–632.
68. Burdin N, Rousset F, Banchereau J. B-cell-derived IL-10: production and function. Methods. 1997. 11:98–111.
69. Blair PA, Noreña LY, Flores-Borja F, Rawlings DJ, Isenberg DA, Ehrenstein MR, Mauri C. CD19+CD24hiCD38hi B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic lupus erythematosus patients. Immunity. 2010. 32:129–140.
70. Tsitoura DC, Yeung VP, DeKruyff RH, Umetsu DT. Critical role of B cells in the development of T cell tolerance to aeroallergens. Int Immunol. 2002. 14:659–667.
71. Lohr J, Knoechel B, Abbas AK. Regulatory T cells in the periphery. Immunol Rev. 2006. 212:149–162.
72. Stohl W, Elliott JE. Differential human T cell-dependent B cell differentiation induced by staphylococcal superantigens (SAg). Regulatory role for SAg-dependent B cell cytolysis. J Immunol. 1995. 155:1838–1850.
73. Fujio K, Okamura T, Yamamoto K. The family of IL-10-secreting CD4(+) T cells. Adv Immunol. 2010. 105:99–130.
74. Zhang L, Yi H, Xia XP, Zhao Y. Transforming growth factor-β: an important role in CD4(+)CD25(+) regulatory T cells and immune tolerance. Autoimmunity. 2006. 39:269–276.
75. Matsushita T, Horikawa M, Iwata Y, Tedder TF. Regulatory B cells (B10 cells) and regulatory T cells have independent roles in controlling experimental autoimmune encephalomyelitis initiation and late-phase immunopathogenesis. J Immunol. 2010. 185:2240–2252.
76. Groux H, O'Garra A, Bigler M, Rouleau M, Antonenko S, de Vries JE, Roncarolo MG. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997. 389:737–742.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download