Abstract
Autoimmune progesterone dermatitis is a rare autoimmune response to endogenous progesterone that usually occurs in fertile females. Cutaneous or mucosal lesions develop cyclically during the luteal phase of the menstrual cycle when progesterone levels are elevated. Symptoms usually start 3-10 days before menstruation and resolve 1-2 days after menstruation ceases. We report the case of a 48-year-old woman with intermittent eczematous skin lesions of the legs, forearms, and buttocks. She was diagnosed with allergic contact dermatitis, and topical steroids were prescribed. Her skin eruptions waxed and waned for 6 years and were associated with her menstrual cycle. We performed an intradermal test using progesterone, which was positive, and prescribed gonadotropin-releasing hormone analogues monthly for 3 months. The patient's skin lesions improved, confirming the diagnosis. Autoimmune progesterone dermatitis should be included in the differential diagnosis of recurrent eczema that is refractory to treatment in women of child-bearing age.
Autoimmune progesterone dermatitis was first described by Géber1 and is a rare disease caused by an autoimmune response to endogenous progesterone in women of child-bearing age. Skin lesions occur periodically during the luteal phase of the menstrual cycle due to increases in progesterone.2,3 Patients with eczematous skin lesions are frequently misdiagnosed with eczematoid dermatitis or allergic contact dermatitis, leading to delays in treatment. We report a case of autoimmune progesterone dermatitis misdiagnosed as allergic contact dermatitis that had been treated without improvement.
A 48-year-old woman visited our clinic with a 6-year history of eczematous skin lesions on the pretibial areas, forearms, elbows, and buttocks bilaterally, with fluctuating clinical symptoms. She had been treated for allergic contact dermatitis without any improvement. There was no medical or family history of allergic disease. She had a 6-month history of oral contraceptive use in her 20s, but had not taken other medications since then. She was twice admitted to hospital for aggravation of her skin lesions. On her last admission, skin biopsies revealed findings suggestive of subacute eczematous dermatitis (Fig. 1A) and an inflammatory cell infiltrate, including eosinophils (Fig. 1B), which led to a diagnosis of allergic contact dermatitis without any identifiable causes.
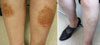
The physical examination revealed multiple eczematous lesions ranging from the size of a small coin to 12 × 8 cm (Fig. 2A). A complete blood cell count showed hemoglobin 9.8 g/dL, hematocrit 29.5%, white blood cells 7,890/µL (eosinophils 690/µL) and platelets 505,000/µL. Liver and thyroid function tests were within normal limits. Her progesterone level was 7.84 ng/mL (3.3-25.0 ng/mL) in the luteal phase.
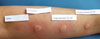
Skin prick tests were negative for representative inhalant allergens, and the serum total IgE level was 22.5 IU/mL. Since the aggravation of her skin lesions was associated with her menstrual cycle, we performed an intradermal test using 50 mg/mL progesterone (Taiyu Progesterone®, Jaytech Biogen, Seoul, Korea) at dilutions of 1:10 and 1:1. Histamine was used as a positive control, and normal saline was used as a negative control. The immediate reaction was read 15 minutes after intradermal injection of the test solutions, and the late reaction was read at 48 hours. The results revealed a positive immediate response (Fig. 3), but no late response. Since the patient was 48 years old and near menopause, she was given three doses of gonadotropin- releasing hormone (GnRH) at 1-month intervals. Subsequently, she entered menopause, and her skin lesions resolved completely (Fig. 2B).
Since Géber1 first described autoimmune progesterone dermatitis in 1921, about 50 cases have been reported in the international literature. In the Korean literature, Hur and Chun3 reported one case of autoimmune progesterone dermatitis and Lee et al.4 presented another case in which circulating autoantibody was identified.
The pathogenesis of autoimmune progesterone dermatitis remains unclear.5 It is proposed that after exposure to exogenous progesterones, especially oral contraceptives or intrauterine devices, sensitized presenting cells and T helper 2 lymphocytes generate specific IgE antibodies, which then cause skin lesions via type 1 hypersensitivity reaction as progesterone levels rise.6 This hypothesis is supported by findings of eosinophilia in the peripheral blood and dense perivascular eosinophilic infiltration around vessels on skin biopsy.7 In our patient, the peripheral eosinophil count was elevated at 690/µL, and eosinophilic infiltration was observed on skin biopsy (Fig. 1B). Intradermal tests with progesterone show both immediate reactions (within 30 minutes) and delayed reactions (24-48 hours later) and may indicate both types I and IV hypersensitivity reactions.8 Only an immediate reaction was observed in our patient, although we did not perform a progesterone patch test to evaluate a type IV reaction. The presence of anti-progesterone antibodies suggests other pathogenic mechanisms,9 including type III hypersensitivity reaction to antigen-antibody complexes that are deposited in the skin, which could induce dermatitis, as progesterone secretion increases before and after menstruation.10 Since this antibody is not detected in all patients, this hypothesis only partially explains the pathogenesis.11 We did not test for anti-progesterone antibodies in our case.
The typical clinical symptoms of autoimmune progesterone dermatitis are skin lesions that develop 3-10 days before menstruation and persist up to 1-2 days after the end of the menstrual cycle, with recurrent cyclic aggravation, closely related to the serum progesterone concentration.12 In our patient, the skin lesions worsened 1 week before menstruation and improved 2-3 days after the end of the menstrual cycle. The skin lesions were associated with urticaria, erythema multiforme, eczema, itching, and vesicular lesions.2 Since eczematous skin lesions are frequently accompanied by itching and are chronic, with frequent recurrences despite medical treatment, they can be misdiagnosed as contact dermatitis, as in our case. In cases of allergic contact dermatitis, eczematous skin lesions occur on the face, neck, wrists, and flexural areas of the lower extremities, and the condition can be diagnosed with a patch test.13
The diagnostic criteria for autoimmune progesterone dermatitis proposed by Warin14 are as follows: (1) skin lesions related to the menstrual cycle; (2) positive response to intradermal testing with progesterone; and (3) symptomatic improvement after inhibiting progesterone secretion by suppressing ovulation. In the intradermal test, immediate and late reactions may occur, so the reactions should be monitored for up to 24-48 hours after allergen injection.1,11
Autoimmune progesterone dermatitis is not very responsive to antihistamines and steroids.15 Therefore, treatment modalities that inhibit progesterone secretion by suppressing ovulation are used widely. GnRH analogues and tamoxifen can successfully treat this disease by suppressing menstruation, but tamoxifen may induce venous thromboembolism and cataract formation.16 For cases that are refractory to medical treatment, bilateral oophorectomy can be considered definitive treatment.11,17
In conclusion, we report a case of autoimmune progesterone dermatitis that was initially diagnosed as allergic contact dermatitis and treated without symptomatic improvement. The patient was diagnosed after a positive response to an intradermal test with progesterone and was successfully treated with GnRH analogues. Autoimmune progesterone dermatitis should be included in the differential diagnosis of recurrent eczema that is refractory to treatment in women of child-bearing age.
Figures and Tables
Fig. 1
(A) The biopsy specimen from the left lower leg consisted of an ellipsoid skin sample with underlying subcutaneous tissue. The hematoxylin and eosin stained histologic section was consistent with acute to subacute eczematous dermatitis (H&E stain, ×40). (B) The histologic section shows an inflammatory cell infiltration around follicular and perivascular tissues with increased dermal eosinophils (H&E stain, ×200).

References
1. Géber H. IV. Einige Daten zur Pathologie der Urticaria menstruationalis. Dermatologische Zeitschrift. 1921. 32:143–150.
2. Snyder JL, Krishnaswamy G. Autoimmune progesterone dermatitis and its manifestation as anaphylaxis: a case report and literature review. Ann Allergy Asthma Immunol. 2003. 90:469–477.
3. Hur W, Chun SI. Autoimmune progesterone dermatitis. Korean J Dermatol. 1993. 31:775–779.
4. Lee CW, Yoon KB, Yi JU, Cho SH. Autoimmune progesterone dermatitis. J Dermatol. 1992. 19:629–631.
5. Wilkinson SM, Beck MH, Kingston TP. Progesterone-induced urticaria--need it be autoimmune? Br J Dermatol. 1995. 133:792–794.
6. Schoenmakers A, Vermorken A, Degreef H, Dooms-Goossens A. Corticosteroid or steroid allergy? Contact Dermatitis. 1992. 26:159–162.
7. Mittman RJ, Bernstein DI, Steinberg DR, Enrione M, Bernstein IL. Progesterone-responsive urticaria and eosinophilia. J Allergy Clin Immunol. 1989. 84:304–310.
8. Baptist AP, Baldwin JL. Autoimmune progesterone dermatitis in a patient with endometriosis: case report and review of the literature. Clin Mol Allergy. 2004. 2:10.
9. Hart R. Autoimmune progesterone dermatitis. Arch Dermatol. 1977. 113:426–430.
10. Miura T, Matsuda M, Yanbe H, Sugiyama S. Two cases of autoimmune progesterone dermatitis. Immunohistochemical and serological studies. Acta Derm Venereol. 1989. 69:308–310.
11. Shelley WB, Preucel RW, Spoont SS. Autoimmune progesterone dermatitis. Cure by oophorectomy. JAMA. 1964. 190:35–38.
12. Burstein M, Rubinow A, Shalit M. Cyclic anaphylaxis associated with menstruation. Ann Allergy. 1991. 66:36–38.
13. Belsito DV. The diagnostic evaluation, treatment, and prevention of allergic contact dermatitis in the new millennium. J Allergy Clin Immunol. 2000. 105:409–420.
14. Warin AP. Case 2. Diagnosis: erythema multiforme as a presentation of autoimmune progesterone dermatitis. Clin Exp Dermatol. 2001. 26:107–108.
15. Stephens CJ, Wojnarowska FT, Wilkinson JD. Autoimmune progesterone dermatitis responding to Tamoxifen. Br J Dermatol. 1989. 121:135–137.
16. Yee KC, Cunliffe WJ. Progesterone-induced urticaria: response to buserelin. Br J Dermatol. 1994. 130:121–123.
17. Ródenas JM, Herranz MT, Tercedor J. Autoimmune progesterone dermatitis: treatment with oophorectomy. Br J Dermatol. 1998. 139:508–511.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download