Abstract
The β2 integrins are expressed exclusively on leukocytes and participate in many immune and inflammatory processes. This subfamily comprises four heterodimeric glycoproteins with a common β-subunit, designated β2 (CD18). Spontaneous mutations of the CD18 gene result in leukocyte adhesion deficiency type I (LAD-I). Low level of CD18 expression has also been implicated in the pathogenesis of psoriasis. We here describe a child with recurrent skin infections without pus formation, persistent gingivitis and periodontitis. His blood counts showed persistent leukocytosis (neutrophilia). CD11b expression was defective on neutrophils, while that of CD18 was normal. So, our patient represents a mild variant of LAD-I with possible dysfunctional CD18. Moreover, he developed psoriasis with reduced CD18 expression on CD4+ T-cells. Psoriasiform dermatitis has been described before in association with LAD-I, however, clinically and histologically confirmed psoriasis in association with LAD-I has been described only in CD18 hypomorphic mice. Therefore, our patient represents the first clinically and histopathologically documented association between LAD-I and psoriasis in humans. It lends support to the role of β2 integrins in the etiopathogenesis of psoriasis.
The β2 integrins are expressed exclusively on leukocytes and participate in many immune and inflammatory processes. This subfamily comprises four heterodimeric glycoproteins with a common β-subunit, designated β2 (CD18).1 In humans, spontaneous mutations of the CD18 gene (chromosome 21q22.3) result in the disease: leukocyte adhesion deficiency type I (LAD-I).2 This is a rare autosomal recessive disease characterized by leukocytosis, recurrent infections without pus formation and poor wound healing. Gingivitis and periodontitis are constant features leading to gingival proliferation and loss of teeth.3
Low-level CD18 expression is thought to be important in the development of psoriasis.4 However, expression of integrins on the cell membrane is not a guarantee of their ability to function as adhesion receptors.5
We here describe a child with a mild variant of LAD-I who developed psoriasis with variable expression of CD18 on neutrophils and CD4+ T-cells.
A 6-year-old Egyptian boy of consanguineous parents presented with fever and multiple skin swellings since the age of one year. These swellings finally ulcerated oozing reddish fluid discharge but with no pus. They tended to heal slowly with scarring after prolonged courses of antibiotics. He also suffered frequent attacks of gingival inflammation with repeated loss of teeth, diarrhea, and chest infections with repeated hospital admissions. There was no history of bleeding tendency.
The child had normal developmental history. There was no history of delayed umbilical stump separation. He had a history of three sib deaths at the ages of one year, two years and five months; all because of severe gastroenteritis with similar recurrent skin infections. His parents and three living sibs were totally free.
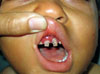
On physical examination he was feverish (38.5℃) and moderately pale. His height and weight were at the 50th percentile for age. He had gingivitis, periodontitis and dental caries (Fig. 1). There were skin abscesses with minimal redness and warmth, some oozing serosanguineous discharge but not pus. Multiple skin scars were observed. Otherwise, he was clinically free.
Laboratory evaluation showed persistent leukocytosis (60×103/µL) with neutrophilia (42×103/µL), microcytic hypochromic anemia (hemoglobin, 8.5 g/dL; MCV, 72 fl) with mild thrombocytosis (520×103/µL). His erythrocyte sedimentation rate was 35 mm/hr and the C-reactive protein was 96 mg/dL. Culture from these abscesses revealed growth of staphylococcus aureus. The bleeding time was normal.
Flow cytometrically assessed, CD11b expression on neutrophils was reduced: 12.9% (93% in healthy control), while that of CD18 was 96% (98% in healthy control). Serum IgG (1,450 mg/dL), IgA (243 mg/dL), IgM (227 mg/dL) and IgE (25 IU/mL) were normal for age. In view of these data, the patient was diagnosed as having a mild variant of LAD-1.
Following control of his acute condition, the child was placed prophylactically on sulfamethoxazole-trimethoprim which decreased the frequency and severity of infections.
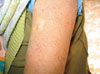
One year ago, he experienced psoriasiform dermatitis (erythematous scaly plaques with well-defined margins) over the trunk, face and flexural areas of the extremities; his nails were free. The scales were silvery white; both Auspitz's sign and Grattage test were positive. So, he fulfilled the clinical diagnostic criteria for psoriasis (Fig. 2) and the skin biopsy confirmed the diagnosis (Fig. 3). Flowcytometric assessment of CD18 on CD4+ T-lymphocytes was done and revealed defective expression: 8% (30.6% in normal control). Topical steroids and zinc oxide led to improvement of psoriasis with residual hypopigmentation.
The severity of infections among LAD-I patients appears to be directly related to the degree of CD18 deficiency. Patients with less than 1% of the normal surface expression exhibit a severe form of disease often leading to death in infancy, whereas patients with some surface expression of CD18 (2.5-10%) manifest a moderate to mild phenotype with fewer serious infections and survival into adulthood.1 However, several LAD-I variants were reported in which there were defects in β2-integrin adhesive functions despite normal surface expression of CD18.6,7
The presence of mild LAD-I phenotype in the face of decreased CD11b expression and almost normal CD18 expression on neutrophils led to the speculation that our patient has defective β2-integrin function. However, this remains to be confirmed whenever tests for β2-integrin function become available in Egypt.
Psoriasis is a common skin disease that takes many clinical forms.8 Auspitz's sign and the Grattage test are pathognomonic of psoriasis.9 Dilated blood vessels, regular epidermal hyperplasia, and presence of Munro microabscess and/or Kogoj's abscess have been described as the most constant or characteristic histopathological features of psoriasis.10 Hence, the clinical and histological findings in our patient were diagnostic of psoriasis.
Development of psoriasis in our patient gives insight onto the role of β2 integrins in the etiopathogenesis of psoriasis. β2 integrins are crucial for the adjustment of antigen-dependent activation threshold. Low level expression of the β2 integrins may lead to deficiency in the numbers and/or functional activity of a population of CD4+ T-cells that normally suppress the development of skin disease in PL/J mouse.11
Psoriasiform dermatitis was described in an adult patient with congenital deficiency of glycoproteins; the authors suggested that it could be an initial stage of psoriasis.12 However, the diagnostic clinical criteria and the nature of the elongation of the rete ridges were not described.
In a CD18 hypomorphic polygenic PL/J mouse model, the severe reduction of CD18 to 2%-16% of wild type levels led to the development of a psoriasiform skin disease which strongly resembles human psoriasis clinically, histologically, and in its response to therapy. Such dermatitis has not been reported in cattle or humans with spontaneous CD18 deficiency resulting in the disease LAD-I.13
In LAD-I, analysis at the gene level revealed a degree of heterogeneity. A number of point mutations were reported, some of which lead to the biosynthesis of defective proteins with single amino acid substitutions, while others lead to splicing defects, resulting in the production of truncated and unstable proteins.14
Our patient represents a rare association of LAD-I and clinically and histopathologically documented psoriasis in humans supporting the role of β2-integrins in the pathogenesis of psoriasis. We assume that this patient has allelic heterogeneity, which accounts for the variable expression of CD18 on neutrophils and T-cells, with possible compromised function of CD18 to be confirmed whenever these tests become available in Egypt.
Figures and Tables
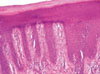
Fig. 3
Skin biopsy findings. The epidermis shows hyperkeratosis, parakeratosis, hypogranulosis and acanthosis with regular elongation of the rete ridges (psoriatic epidermal hyperplasia). The dermis shows edema and mixed dermal inflammatory infiltrate together with dilation of the papillary capillaries.
References
1. Etzioni A, Harlan JM. Ochs HD, Smith CIE, Puck JM, editors. Cell adhesion and leukocyte adhesion defects. Primary immunodeficiency diseases: a molecular and genetic approach. 2007. 2nd ed. New York: Oxford University Press;551.
2. Solomon E, Palmer RW, Hing S, Law SK. Regional localization of CD18, the beta-subunit of the cell surface adhesion molecule LFA-1, on human chromosome 21 by in situ hybridization. Ann Hum Genet. 1988. 52:123–128.
3. Kilic SS. Leukocyte adhesion deficiency in a case presenting as septic arthritis. Int Pediatr. 2002. 17:96–97.
4. Lebwohl M. Psoriasis. Lancet. 2003. 361:1197–1204.
5. Diamond MS, Springer TA. The dynamic regulation of integrin adhesiveness. Curr Biol. 1994. 4:506–517.
6. Hogg N, Stewart MP, Scarth SL, Newton R, Shaw JM, Law SK, Klein N. A novel leukocyte adhesion deficiency caused by expressed but nonfunctional beta2 integrins Mac-1 and LFA-1. J Clin Invest. 1999. 103:97–106.
7. Mathew EC, Shaw JM, Bonilla FA, Law SK, Wright DA. A novel point mutation in CD18 causing the expression of dysfunctional CD11/CD18 leucocyte integrins in a patient with leucocyte adhesion deficiency (LAD). Clin Exp Immunol. 2000. 121:133–138.
8. Farber EM, Nall L. Childhood psoriasis. Cutis. 1999. 64:309–314.
9. Hellgren L. Psoriasis: the prevalence in sex, age and occupational groups in total populations in Sweden. Morphology, inheritance and association with other skin and rheumatic diseases. 1967. Stockholm: Almqvist and Wiksell;134–145.
10. Toussaint S, Hideko K. Elder D, Elenitsas R, Jaworsky C, Johnson B, editors. Non infectious erythematous popular and squamous diseases of the skin. Lever's histopathology of the skin. 1997. 8th ed. Philadelphia: Lippincott-Raven;156–163.
11. Barlow SC, Xu H, Weaver CT, Lindsey JR, Schoeb TR, Bullard DC. Development of dermatitis in CD18-deficient PL/J mice is not dependent on bacterial flora, and requires both CD4+ and CD8+ T lymphocytes. Int Immunol. 2004. 16:345–351.
12. van de Kerkhof PC, Weemaes CM. Skin manifestations in congenital deficiency of leucocyte-adherence glycoproteins (CDLG). Br J Dermatol. 1990. 123:395–401.
13. Kess D, Peters T, Zamek J, Wickenhauser C, Tawadros S, Loser K, Varga G, Grabbe S, Nischt R, Sunderkötter C, Müller W, Krieg T, Scharffetter-Kochanek K. CD4+ T cell-associated pathophysiology critically depends on CD18 gene dose effects in a murine model of psoriasis. J Immunol. 2003. 171:5697–5706.
14. Kishimoto TK, Hollander N, Roberts TM, Anderson DC, Springer TA. Heterogeneous mutations in the beta subunit common to the LFA-1, Mac-1, and p150,95 glycoproteins cause leukocyte adhesion deficiency. Cell. 1987. 50:193–202.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download