Abstract
Hydroxyapatite is commonly used as a filler to replace amputated bone or as a coating to promote bone ingrowth into prosthetic implants. Many modern implants, such as hip replacements and dental implants, are coated with hydroxyapatite. We report a patient with occupational asthma due to hydroxyapatite, proven by a specific inhalation challenge, who experienced an early asthmatic reaction after exposure to hydroxyapatite, without increased airway responsiveness to methacholine despite an increased eosinophil count in the peripheral blood. A 38-year-old male dental implant worker visited our allergy department for the evaluation of occupational asthma. He had treated dental implant titanium surfaces with hydroxyapatite for 1.5 years. One year after starting his employment, he noticed symptoms of rhinorrhea, paroxysmal cough, and chest tightness. His symptoms were aggravated during and shortly after work and subsided several hours after work. When he stopped working for 2 months because of his chest symptoms, he became asymptomatic. After restarting his work, his symptoms reappeared and were aggravated. A methacholine bronchial challenge test had a negative response. The following day, a specific bronchial provocation test with wheat powder was negative. On the third day, a specific bronchial provocation test with hydroxyapatite powder produced an early asthmatic response. On the fourth day, a methacholine bronchial challenge test was negative. Further studies are needed to evaluate the exact pathogenetic mechanism of hydroxyapatite-induced occupational asthma.
While symptomatic patients with occupational asthma (OA) have non-specific airway hyper-responsiveness (NSAH), their NSAH may disappear after exposure to the offending agent ceases. Conversely, the induction of an asthmatic reaction after specific bronchial provocation testing is often associated with an increase in NSAH.1,2 This report is about a dental implant worker with symptoms of OA. His job involved blasting titanium for dental implants with hydroxyapatite. A specific bronchial provocation test with hydroxyapatite showed an early asthmatic response. However, no NSAH to methacholine was seen before or after the hydroxyapatite provocation test. To our knowledge, this is the first report of hydroxyapatite-induced OA, and it was not accompanied by increased NSAH after a specific bronchial provocation test.
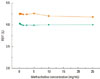
The patient was a 38-year-old male, non-smoker, who had been employed in a dental implant factory for 1.5 years. His work consisted of treating titanium surfaces with hydroxyapatite in a blasting machine. One year after starting his job, the patient developed rhinorrhea, a paroxysmal cough, and chest tightness. His symptoms were aggravated during and shortly after work and subsided several hours after work. When the patient stopped working for 2 months because of chest symptoms, he became asymptomatic. After restarting his work, the symptoms reappeared and were aggravated. At presentation, a blood differential count including eosinophils (555 cells/µL), serum biochemistry, and chest and paranasal sinus radiographs showed no abnormalities. The total IgE was 275 kU/L (normal range 0-114 kU/L). Skin prick tests were negative for 50 common inhalant allergens. NSAH to methacholine was not noted. To evaluate the causal relationship, a specific bronchial provocation test with hydroxyapatite powder was performed. After inhaling the hydroxyapatite powder for 45 min, the forced expiratory volume in 1 second (FEV1) decreased to 20% of the baseline value (Fig. 1). The next day, no NSAH to methacholine was seen (Fig. 2). The peripheral blood examination revealed a slightly increased eosinophil level to 875 cells/µL after the specific bronchial provocation test with hydroxyapatite. The patient was diagnosed as having hydroxyapatite-induced OA without an increased NSAH to methacholine.
This is a very unusual case of OA with an early asthmatic reaction to a specific allergen challenge without NSAH before or after the specific allergen provocation test. The occurrence of an asthmatic reaction without NSAH has been described in some cases.3,4 Similar to two reported cases, our subject had negative results on methacholine bronchial challenge tests before and after the asthmatic reaction. When he visited our department for evaluation of his chest symptoms, the patient had already been off work for approximately 1 month. This might explain the negative NSAH before the specific bronchial provocation test. We also performed specific bronchial challenge tests with a placebo (wheat flour) to rule out a non-specific irritative reaction. This test was negative, so we were able to exclude the possibility of a false positive response to hydroxyapatite. However, we did not perform a specific bronchial provocation test with hydroxyapatite in patients with asthma who had not been exposed to hydroxyapatite. This is a limitation of our study.
Serial measurement of the peak expiratory flow with the subject at work and away from work would be useful for obtaining objective information to confirm OA.5 However, the patient would not agree to peak-expiratory flow rate monitoring. Nevertheless, his chest symptoms were closely related to his work hours, and subsided while he was on holiday.
In this study, there was no NSAH to methacholine after the specific bronchial provocation test with hydroxyapatite. We used the five (total lung capacity)-breath dosimeter method, as modified from a method described by the American Academy of Allergy and Immunology.6 In contrast to the modified tidal breathing method of Cockcroft et al.7, this method may cause bronchoprotection and give a false-negative result. Recent studies performed in large numbers of individuals showed that approximately equivalent results for the two methods are seen in subjects with mild to moderate or greater airway hyperresponsivenss.8 However, the five-breath dosimeter method might protect from bronchoconstriction to methacholine in asthmatics with very mild airway hyperresponsiveness.9,10 These findings may result in the negative response regarding NSAH to methacholine seen in our patient.
The peripheral blood examination showed an increased eosinophil count after the specific bronchial provocation test with hydroxyapatite in this case. This suggests that the occurrence of NSAH is unlikely to be induced by eosinophilic airway inflammation alone. Eosinophilic bronchitis is one of the important diseases characterized by airway inflammation without NSAH.11 It is possible that in subjects with very low baseline responsiveness, a great deal of inflammation is necessary to induce airway responsiveness.
Hydroxyapatite is a naturally occurring mineral form of calcium apatite and is chemically similar to the mineral component of bone and hard tissues in mammals.12 As it is insoluble, we could not perform skin tests or other studies to evaluate the immunological or non-immunological mechanisms. Further studies are required to evaluate the chemical and immunological characteristics of hydroxyapatite.
Although this case has a few limitations regarding the diagnosis of OA, we would like to present it as a very rare case of OA induced by hydroxyapatite without accompanying NSAH to methacholine. Further efforts are required to identify other cases of OA induced by hydroxyapatite and evaluate their pathogenetic mechanisms.
Figures and Tables
Fig. 1
Dose-response curves for the specific bronchial provocation tests with hydroxyapatite and placebo (rectangles, hydroxyapatite; circles, placebo).
FEV1, forced expiratory volume in 1 second.

Fig. 2
Methacholine bronchial challenge tests on days 1 (top, orange rectangles) and 4 (bottom, green circles). On day 1, the baseline FEV1 was 4.31 L and the minimum FEV1 after methacholine inhalation was 4.18 L. On day 4, the baseline FEV1 was 4.0 L and the minimum FEV1 after methacholine inhalation was 3.99 L.
FEV1, forced expiratory volume in 1 second.
References
1. Bernstein DI, Campo P, Baur X. Bernstein IL, Chan-Yeung M, Malo JL, Bernstein DI, editors. Clinical assessment and management of occupational asthma. Asthma in the workplace. 2006. 3rd ed. New York: Taylor & Francis;161–178.
2. Malo JL, Chan-Yeung M. Occupational asthma. J Allergy Clin Immunol. 2001. 108:317–328.
3. Lemiere C, Weytjens K, Cartier A, Malo JL. Late asthmatic reaction with airway inflammation but without airway hyperresponsiveness. Clin Exp Allergy. 2000. 30:415–417.
4. Banks DE, Barkman HW Jr, Butcher BT, Hammad YY, Rando RJ, Glindmeyer HW 3rd, Jones RN, Weill H. Absence of hyperresponsiveness to methacholine in a worker with methylene diphenyl diisocyanate (MDI)-induced asthma. Chest. 1986. 89:389–393.
5. Dykewicz MS. Occupational asthma: current concepts in pathogenesis, diagnosis, and management. J Allergy Clin Immunol. 2009. 123:519–528.
6. Chai H, Farr RS, Froehlich LA, Mathison DA, McLean JA, Rosenthal RR, Sheffer AL, Spector SL, Townley RG. Standardization of bronchial inhalation challenge procedures. J Allergy Clin Immunol. 1975. 56:323–327.
7. Cockcroft DW, Killian DN, Mellon JJ, Hargreave FE. Bronchial reactivity to inhaled histamine: a method and clinical survey. Clin Allergy. 1977. 7:235–243.
8. Cockcroft DW, Davis BE, Todd DC, Smycniuk AJ. Methacholine challenge: comparison of two methods. Chest. 2005. 127:839–844.
9. Todd DC, Davis BE, Hurst TS, Cockcroft DW. Dosimeter methacholine challenge: comparison of maximal versus submaximal inhalations. J Allergy Clin Immunol. 2004. 114:517–519.
10. Allen ND, Davis BE, Hurst TS, Cockcroft DW. Difference between dosimeter and tidal breathing methacholine challenge: contributions of dose and deep inspiration bronchoprotection. Chest. 2005. 128:4018–4023.
11. Gibson PG, Dolovich J, Denburg J, Ramsdale EH, Hargreave FE. Chronic cough: eosinophilic bronchitis without asthma. Lancet. 1989. 1:1346–1348.
12. Hayes CW, Conway WF. Calcium hydroxyapatite deposition disease. Radiographics. 1990. 10:1031–1048.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download