Abstract
Purpose
Patients with a history of allergic reaction to penicillin, but with no detectable specific IgE, are common and pose a dilemma. Challenge tests are considered to be the diagnostic gold standard. The aim of this study was to identify subgroups of patients with very low risk for reactions who could be safely tested using a more rapid and simple procedure.
Methods
A total of 580 consecutively referred adult patients with a history of non-serious cutaneous allergic reactions to penicillin, but with no IgE, were challenged with therapeutic doses of penicillin V (phenoxymethylpenicillin), penicillin G (benzylpenicillin), or both.
Results
Only 14 of 580 patients had a positive challenge test. In 11 of the 14, a reaction to challenge occurred within 2 hours, and none were anaphylactic. The year of the original reaction was known for 555 patients; a positive challenge was seen in only 0.4% of those with an original reaction >15 years before challenge, but in 4.6% of those with a more recent original reaction (P=0.001). Onset of a reaction within the first day of the original exposure was a predictive factor for a positive challenge (P=0.001) in patients challenged within 15 years of the original reaction.
Conclusions
Among suspected penicillin-allergic patients with non-severe skin reactions and no detectable specific IgE, the subgroup of patients who originally reacted more than 15 years previously had very low risk for reacting to a challenge. The risk was higher in patients with a more recent original reaction, especially if the symptoms had occurred within the first day of exposure.
Patients with a history of allergic reaction to penicillin, but with no detectable specific IgE, are common and pose a dilemma. The incidence of allergic reactions to drugs is difficult to determine. In 2001, the authors of a large review of the international literature found adverse reactions to drugs in 1.8-15.1% of hospitalized patients.1 Regarding penicillin, a retrospective study from 2004 that involved 3,375,162 patients who received a prescription for penicillin reported an allergy-like reaction in 6,212 (0.18%).2 The Danish Drug Administration has identified 30 cases of fatal anaphylactic shock due to drugs in the Danish population of 5 million people from 1968 to 1990.3 Of these 30 fatal reactions, seven were caused by antibiotics: penicillin in four cases, ampicillin in two, and a sulfa drug in one.
In most European countries, the use of narrow-spectrum penicillins has steadily decreased during the past decades. Although narrow-spectrum penicillins are still the most commonly sold antibiotics in Denmark and Sweden and are still used in Finland, Ireland, the UK, Austria, Germany, and the Netherlands, they are almost obsolete in other countries.4 This may explain why several studies have found reactions to penicillin to be quite rare compared with reactions to, for example, cephalosporins.5,6
Genetic factors such as a family history of reactions to penicillins may also play a role, as recently investigated.7 The parenteral route of administration is thought to cause more allergic reactions.8 Dosage and frequency of administration may also influence the incidence of reactions, but documentation is sparse.
Drug challenge is the only certain way to discriminate between patients with and without future risk for reactions to penicillin. However, because of the potential for severe anaphylactic and other serious reactions, testing must be performed with caution. This makes testing demanding with respect to time and labor. The aim of this study was to identify subgroups of patients with very low risk for reactions who could be safely tested using a more rapid and simple procedure.
At our clinic, controlled challenges with penicillin are performed in the majority of patients referred for suspected allergy (but no IgE) to penicillins. Exceptions are patients who decline this test and those who have had severe skin reactions such as exfolative or bullate reactions, or systemic reactions involving the liver or bone marrow. Most patients are referred by general practitioners, and a few come from other hospital departments, dentists, and private clinics. Doctors are usually aware of our challenge criteria, and thus selection takes place prior to referral. Using the hospital's administrative system, we identified 580 patients (444 females and 136 males) who had been challenged with penicillin V (phenoxymethylpenicillin), penicillin G (benzylpenicillin), or both, between January 1, 2001 and December 31, 2006. Clinical data were collected from the patients' medical notes. The patients ranged in age from 13 to 87 years, with a mean of 39 years (women, 38.9 years; men, 39.2 years).
Approval for data collection and storage was obtained from the Danish Data Protection Agency (2009-41-3271). The regional ethics committee did not find it necessary to approve this retrospective study.
All patients were interviewed by a physician before beginning the challenge. Patients with a history suggestive of an allergic reaction to penicillin (skin rash or angioedema), but no history of other systemic reactions or severe skin reactions as aforementioned, were offered the challenge. All patients were tested for the presence of relevant specific IgE in serum, and the patients testing positive who did not receive the challenge because of a high suspicion of allergy were not included in the study. An ImmunoCAP fluorescence enzyme immunoassay system (Phadia, Uppsala, Sweden) was used for IgE measurements. Standard analyses included those for the allergens penicilloyl G, penicilloyl V, amoxicilloyl, and ampicilloyl, with a cut-off value of 0.35 kUA/L. All patients with no detectable IgE were offered a challenge with penicillin V, penicillin G, or both.
After informed consent was obtained, the patients were challenged with the penicillin suspected of having caused the original reaction. For cases in which this was unknown, penicillin V, penicillin G, or both were chosen, depending on the route of administration. Challenges were performed in the ward, which was equipped with facilities for treatment of anaphylaxis, and patients were observed for at least 2 hours after the last dose. An incremental procedure was applied, with the administration of 1:100, 1:10, and full therapeutic (1 million IE) doses at intervals of 30 to 60 minutes. The dose titration with intravenous penicillin G to the full dose was most often followed by a full oral provocation dose of penicillin V. No placebo was included, as objective symptoms were necessary for a positive challenge. Patients were instructed to report back with any suspicion of late reactions within the days after the challenge.
Statistical analyses were performed using SPSS version 15.0 software (SPSS, Inc., Chicago, IL, USA). The 95% confidence intervals were determined from the Poisson distribution, and P values were calculated using a chi-squared or Fisher's exact test for 2×2 tables. Values of P<0.05 were considered to indicate significance.
During the study period, challenges were performed in 580 patients, and 14 patients (2.4%) (7 females and 7 males) had positive reactions (Table 1). Of these, 11 reactions occurred at the clinic and three were delayed, appearing after the patient had returned home. None fulfilled the criteria of an anaphylactic reaction.
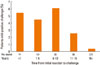
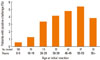
The time between the original reaction and the challenge and the age at the time of the original reaction were analyzed. Positive challenges appeared to occur more often in those with a more recent original reaction (Fig. 1), but were more evenly distributed according to age (Fig. 2). The median time interval between the original reaction and the challenge was 15 years. Among the 280 patients who had experienced an original reaction within the previous 15 years, 13 (4.6%) had a positive challenge. In contrast, only one (0.4%) of the 275 patients with an original reaction that had occurred more than 15 years earlier showed a positive reaction to the challenge (P=0.001).
The aim of this investigation was to identify predictive parameters for the outcome of the challenge, and the time between the original reaction and the challenge appeared to be a strong predictive factor. Therefore, the subgroup of patients with a reported original reaction within the 15 years prior to the challenge was chosen for subsequent analyses.
Seventy-nine of the 280 patients recalled with certainty having received penicillin V before the original reaction. Of these, 7.1% (6/79) had a positive challenge. Among the patients who reported symptom onset within 1 day after the original dose, 11% (7/64) reacted positively to the challenge, in contrast to only 1% (1/137) of those reporting a later reaction (P=0.001) (Table 2). Itching was investigated separately, and none of the patients who reported the absence of an itching reaction had a positive challenge result (Table 3). However, a large portion of the patients did not remember whether there had been itching. Eight (6.1%) of 134 patients with a generalized rash reacted to the challenge, compared with one (2.8%) of 36 patients with localized reactions (P=0.29). Of the 280 patients with a more recent original reaction, 108 did not report a rash or did not remember the extent of it. For the 135 patients with information about the duration of the original reaction, no significant difference in reaction rate was found between those with symptoms lasting 0-2 days and those with more long-lasting symptoms. A history of allergy to penicillin in first-line relatives showed no significant correlation with the challenge outcome.
The diagnosis of allergy to penicillin is based on clinical history and the results from IgE antibody measurements and skin tests. When these results are not in accordance, a challenge test is necessary for diagnosis. Standardized skin test material has not been available for several years, and skin testing was not performed in the present cohort. A recent study investigating non-immediate reactions in children found skin testing to be of limited value in this type of reaction.9 The intention of the challenge test in the study analyzed here was to identify severe and anaphylactic reactors among the referred patients, and thus the test included only one or two full doses, and not an entire treatment period. As patient selection in the study analyzed here was not limited to only those with a clear history of an immediate reaction, it is possible that some late reactions might have been missed. This could explain why our study, despite not using skin tests, found a low number of positive challenges compared with other studies. Another explanation could be a tendency toward selecting patients with milder reactions prior to referral.
The suspected rate of allergy to penicillin varies among different populations and different geographical locations, and the same appears to be true for the percentage of challenge-positive patients with no IgE sensitization. The present study represents one of the largest series of standardized challenge testing in suspected penicillin-allergic patients and allows for more conclusions regarding the possible risks and predictive factors for challenge testing.
Based on the data from this study, a patient with a history of a mild reaction to penicillin that occurred more than 15 years previously and with no detectable serum IgE antibodies to penicillin V, penicillin G, amoxicillin, or ampicillin would have only a 0.4% risk for reacting when given penicillin V or G in a clinical setting. In another study that included adult patients and challenge testing at a time closer to the original reaction, the mean age at the original reaction did not differ between patients with a positive challenge and those with a negative challenge.10 However, as in our study, the time interval between the original reaction and the challenge showed a significant difference between the positive and negative reactors, with a mean of 385 days for positive outcomes compared with 769 days for negative outcomes. Thus, the time span from the original reaction to the challenge may be a stronger predictive factor than patient age at the original reaction. Therefore, we agree that much earlier reactions to penicillin are unlikely to be confirmed at challenge, as recently suggested.10
Two prospective studies found incidences of 8.4% and 7.6% positive challenges within a skin test-negative population of patients with a history of a physician-diagnosed reaction.11,12 One reason for the higher reaction rates in those studies compared with ours could be that all of the patients in the two earlier studies were challenged within 15 years of their original reaction, and most within a much shorter time span. Another study from 2002, which included only skin test-negative patients with no detectable specific IgE, found 55% of 89 patients to be positive on challenge with β-lactams.13 All of the patients in that study originally reacted within 1 hour after drug administration, and patients with an original reaction that occurred more than 15 years prior to the study were not included. Our results indicate that a short interval between the initiation of exposure and the reaction is an important predictor for a positive challenge; an original reaction occurring on the first day of exposure increased the risk for a positive challenge more than 10-fold.
A large number of patients who believe they are allergic to penicillin do not have a reaction upon challenge with penicillin. This could be due to various possible reasons, including (1) the original reaction was not an allergic reaction; (2) the original reaction was to another drug administered at the same time; (3) the original reaction was to another drug, but was recalled as penicillin; or (4) the patient became tolerant to penicillin. It is known that the level of specific IgE to penicillin decreases over time with no exposure.14,15 The much lower rate of allergy in our patients who originally reacted more than 15 years earlier suggests that the patients allergic to penicillin could outgrow their allergy, but not until after the level of specific IgE becomes undetectable. We did not perform systematic follow-ups, but have not been contacted by any patients who reacted when reintroduced to penicillin at a later date, although a recent review found re-sensitization rates of 0.9% to 27.9%.16
Even though more females were tested in our study, an equal number of males and females responded positively to the challenge. In Denmark, women had 47% more contacts with general practitioners and received 37% more defined daily doses of anti-infectious drugs (Anatomical Therapeutic Chemical Classification, ATC group J) than did men in 2005.17 The more frequent exposure to antibiotics might have caused a higher frequency of allergic women, but this was not confirmed in our study, perhaps because of the small number of positives.
In conclusion, the data from this study suggest that patients with an original reaction more than 15 years previously, without a history of serious rash or systemic reactions, and with no specific IgE can be safely challenged with penicillin in a setting with treatment for anaphylaxis at hand, but without titration of doses and without intravenous access. The time of symptom onset gave a good indication of the outcome of the challenge in patients with an original reaction within the previous 15 years. Furthermore, in this subgroup, no positive challenges were observed in patients reporting the original reaction to be without itch. However, this information was often unavailable in cases with a long time span between reaction and testing. To increase our knowledge, a standardized and more detailed case history and less delay in patient referral are needed in future prospective studies.
Figures and Tables
Fig. 1
Challenge outcomes according to the time interval between the original reaction and the challenge test.
References
1. Demoly P, Bousquet J. Epidemiology of drug allergy. Curr Opin Allergy Clin Immunol. 2001. 1:305–310.
2. Apter AJ, Kinman JL, Bilker WB, Herlim M, Margolis DJ, Lautenbach E, Hennessy S, Strom BL. Represcription of penicillin after allergic-like events. J Allergy Clin Immunol. 2004. 113:764–770.
3. Lenler-Petersen P, Hansen D, Andersen M, Sørensen HT, Bille H. Drug-related fatal anaphylactic shock in Denmark 1968-1990. A study based on notifications to the Committee on Adverse Drug Reactions. J Clin Epidemiol. 1995. 48:1185–1188.
4. Cars O, Mölstad S, Melander A. Variation in antibiotic use in the European Union. Lancet. 2001. 357:1851–1853.
5. Ibia EO, Schwartz RH, Wiedermann BL. Antibiotic rashes in children: a survey in a private practice setting. Arch Dermatol. 2000. 136:849–854.
6. Bigby M, Jick S, Jick H, Arndt K. Drug-induced cutaneous reactions. A report from the Boston Collaborative Drug Surveillance Program on 15,438 consecutive inpatients, 1975 to 1982. JAMA. 1986. 256:3358–3363.
7. Apter AJ, Schelleman H, Walker A, Addya K, Rebbeck T. Clinical and genetic risk factors of self-reported penicillin allergy. J Allergy Clin Immunol. 2008. 122:152–158.
8. Gomes ER, Demoly P. Epidemiology of hypersensitivity drug reactions. Curr Opin Allergy Clin Immunol. 2005. 5:309–316.
9. Blanca-López N, Zapatero L, Alonso E, Torres MJ, Fuentes V, Martínez-Molero MI, Blanca M. Skin testing and drug provocation in the diagnosis of nonimmediate reactions to aminopenicillins in children. Allergy. 2009. 64:229–233.
10. Goldberg A, Confino-Cohen R. Skin testing and oral penicillin challenge in patients with a history of remote penicillin allergy. Ann Allergy Asthma Immunol. 2008. 100:37–43.
11. Messaad D, Sahla H, Benahmed S, Godard P, Bousquet J, Demoly P. Drug provocation tests in patients with a history suggesting an immediate drug hypersensitivity reaction. Ann Intern Med. 2004. 140:1001–1006.
12. Bousquet PJ, Pipet A, Bousquet-Rouanet L, Demoly P. Oral challenges are needed in the diagnosis of β-lactam hypersensitivity. Clin Exp Allergy. 2008. 38:185–190.
13. Torres MJ, Mayorga C, Leyva L, Guzman AE, Cornejo-García JA, Juarez C, Blanca M. Controlled administration of penicillin to patients with a positive history but negative skin and specific serum IgE tests. Clin Exp Allergy. 2002. 32:270–276.
14. Antúnez C, Fernández T, Blanca-Lopez N, Torres MJ, Mayorga C, Canto G, Fernández J, Moya MC, Blanca M. IgE antibodies to betalactams: relationship between the triggering hapten and the specificity of the immune response. Allergy. 2006. 61:940–946.
15. Fernández TD, Torres MJ, Blanca-López N, Rodríguez-Bada JL, Gomez E, Canto G, Mayorga C, Blanca M. Negativization rates of IgE radioimmunoassay and basophil activation test in immediate reactions to penicillins. Allergy. 2009. 64:242–248.
16. Blanca M, Romano A, Torres MJ, Férnandez J, Mayorga C, Rodriguez J, Demoly P, Bousquet PJ, Merk HF, Sanz ML, Ott H, Atanasković-Marković M. Update on the evaluation of hypersensitivity reactions to betalactams. Allergy. 2009. 64:183–193.
17. Vedsted P. Gender differences in the use of health care system. Ugeskr Laeger. 2007. 169:2403–2408.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download