Abstract
Allergic diseases represent a complex innate and adoptive immune response to natural environmental allergens with Th2-type T cells and allergen-specific IgE predominance. Allergen-specific immunotherapy is the most effective therapeutic approach for disregulated immune response towards allergens by enhancing immune tolerance mechanisms. The main aim of immunotherapy is the generation of allergen nonresponsive or tolerant T cells in sensitized patients and downregulation of predominant T cell- and IgE-mediated immune responses. During allergen-specific immunotherapy, T regulatory cells are generated, which secrete IL-10 and induce allergen-specific B cells for the production of IgG4 antibodies. These mechanisms induce tolerance to antigens that reduces allergic symptoms. Although current knowledge highlights the role of T regulatory cell-mediated immunetolerance, definite mechanisms that lead to a successful clinical outcomes of allergen-specific immunotherapy still remains an open area of research.
The main role of the immune system is the ability to distinguish self from non-self while still responding to and neutralizing pathogens. The physiopathology of immune tolerance-related diseases, such as allergy, asthma or autoimmune diseases is complex and influenced by several factors. These include genetic susceptibility, the nature of the antigen that initiates the disease (antigen dose, time of exposure, route of exposure, and its structural characteristics) and possible co-exposure with innate immune response stimulating substances, such as infections and flora bacteria.1
Since allergy is a predominantly Th2 type of immune disorder, one of the essential ways to overcome the deviated immune response has been allergen-specific immunotherapy (SIT) that involves repeated administration of the sensitizing allergens by subcutaneous injection or mucosal route. The induction of peripheral tolerance towards the responsible allergen is the main target in allergen-SIT. After successful immunotherapy, allergen-specific T regulatory cells (Treg) are generated and they suppress proliferative and cytokine responses against allergens.2 In addition, antibody class-switching occurs in B cells that secrete allergen-specific IgG4 instead of IgE which has blocking capacity inhibiting binding between allergen and IgE on mast cells and basophils. This review focuses on mechanism of allergen-SIT and discusses the current approaches in clinical and research perspective of immune tolerance induction in allergic disorders.
Loss of tolerance to certain allergens such as, aeroallergens, foods and insect venom, leads to induction of type I hypersensitivity reactions. The type of immune response influenced by several factors including genetic susceptibility, the nature of antigen which initiates the disease (antigen dose, time of exposure, route of exposure, and structural characteristics), and challenge with infections and bacteria.3 Under these complex stimulus, navie T cells activated by professional antigen-presenting cells (APC) and differentiate into Th1, Th2, Th17 or Th9 cells. For atopic disease, the Th2 arm of immune response is responsible for immunopathology and clinical scene. Once Th2 response is established, the mechanism of allergic disease is further divided into two main phases: first sensitization, and development of memory and later followed by effector phase and tissue injury. In the sensitization phase, allergen-specific CD4+Th2 cells produce IL-4 and IL-13, which induce B cell class-switch into the antibody isotypes of ε immunoglobulin heavy chain and the production of allergen-specific IgE antibody. Later, allergen-specific IgE, binds to high affinity receptor for IgE (FcεRI receptors) on the surface membrane of mast cells and basophils. These series of activations lead to the sensitization of the patients to a specific allergen. Re-exposure to the sensitized allergen leads to the aggregation of receptor-bound IgE molecules and results in the activation and mediator release that lead to the development of clinical symptoms of type I hypersitivity reactions.4,5
Immediate reactions are followed by late phase reactions with the activation of T cells by continuous presence of allergens. Once allergen-specific CD4+Th2 cells are activated, they produce IL-4, IL-5, IL-9, and IL-13, which play crucial role in the maintenance of high allergen-specific IgE levels, stimulate eosinophil progenitors in the bone marrow, induce inflammatory cell influx into inflamed tissue, and induce production of mucus and smooth muscle contraction.3 These events require T cell activation and peripheral T cell tolerance prevents formation of atopic immunopathology in healthy subjects. A continuous interaction with migrating T cells and resident tissue cells takes place and other subsets such as Th1 cells, Th9 cells, Th17 cells, and Th22 cells also play important roles.6-8
Th17 cells represent a newly discovered subset of T lymphocytes and are involved in the pathogenesis of several immune-mediated disorders. IL-17, IL-6, TNF-α, and IL-22 are signature cytokines of Th17 cells and play important roles on tissue pathology in autoimmune disorders as well as allergic disease.9 Recent studies showed the importance of allergen-specific Th17 cells in humans.10 IL-17 is essentially important for the recruitment of neutrophils and expressed in bronchial biopsies, bronchoalveolar lavage fluid and sputum of patients of asthma.11 IL-17A and IL-17F are negative regulators of antigen driven Th2 response.12,13 It has also been demonstrated that anti-IL-17 reduces neutrophilic infiltration in experimental murine asthma models.14 Furthermore, IL-17 increases eosinophilic infiltration and recruitment/survival of airway macrophages.12 Patients with allergic rhinitis revealed high frequency of IL-17-producing T cells.15 Taken together, IL-17 contribute to the differentiation and activation of allergen-specific Th2 cells, influx of eosinophils to target organs, and serum IgE production that provide important clues in the role of Th2 driven allergic response.16
Similar to Th17 cells, Th22 cells were rarely found in PBMCs, while they were clearly detected in T cell population isolated from skin of patients with psoriasis, atopic eczema and allergic contact dermatitis.17 Furthermore, IL-22 synergizes with IL-17 in the induction of proinflammatory cytokines in human bronchial epithelial cells18 and colonic myofibroblasts.19
Dendritic cells (DCs) play a crucial role for detecting innate pathogens and triggering adaptive immune response. Within the subtypes of DCs, plasmacytoid DCs (pDCs) take attention by their surface phenotype, tissue localization, cytokine secretion, and antigen-presentation function,20 and keep important role in the initiation and regulation of immune response and immune tolerance.21 The maturation period influences the role of DCs in the tolerance formation. Especially, semi-mature DC induce tolerance in the immune system, whereas matured DCs mediate expression of interferon (IFN)-γ producing T cells.22 Taken together, distrubiton of DC subsets and their maturation stage seems to be essential in tolerance induction to certain antigens.
Murine oral mucosal dendritic cells (mDCs) express CD11b+/CD11c-/+ at the mucosal/submucosal junction zone, whereas langerhans cells epress CD207+ within the mucosal epithelium and pDCs express B220+/120G8+ in the submucosal region. In a study on sublingual application of OVA, a total clearence of OVA within 15-30 minutes by CD11b+/CD11c-/+ mDCs was shown.23 It seems however that these murine studies are not fully relevant to human in vivo stimulation. In humans, oral mucosal Langerhans cells (oLCs) represent the predominant DC population, however pDCs are virtually absent in oral mucosa. oLCs constitutively express high affinity receptor for IgE, which is absent in classical epidermal Langerhans cells. FcεRI expression is seen during early differentiation period of Langerhans cells as well as other DCs and it possesses a pro-tolerogenic character. Studies clearly demonstrate that oLCs of atopic individuals show increased expression of FcεRI that cooperates with IgE.24 This strategic location of oLCs at suprabasal epithelium layer and increased expression of FcεRI may facilitate binding and processing of allergens in sublingual immunotheraphy (SLIT) period.
The importance and functions of Treg cells to induce tolerance have been explicitly studied during the last 15 years. The major role of Treg cells in immune tolerance was clarified in murine studies directly or adoptive transfer of Treg cells. They prevent or cure numerous T-cell-mediated disease models, including, asthmatic lung inflammation, autoimmune diseases and allograft rejection, by achieving immune tolerance to responsible allergens, self antigens or alloantigens.25 On the other hand, chronic absence or imperfect function of Treg cells may lead a series of immune dysfunction disease, such as hyper IgE syndrome, hyper eosinophilia and autoimmunity in humans, which is normal with appropriate function of Treg cells.26 For an easy understanding, Treg cells may be divided into two main subgroups; The naturally occurring forkhead box P3 (Foxp3)+CD4+ CD25+ regulatory T cells (will be referred as CD4+CD25+Treg cells)27, which develop in the thymus and are present in birth, and the other is inducible Treg cells, which is generated in the periphery under various tolerogenic conditions. Especially, the IL-10-producing T regulatory type 1 (Tr1) cells have been shown to play a key role in allergen tolerance, and can be induced by allergen-SIT in humans.28-31 With recent studies, it is well established that Foxp3 acts as master switch transcription factor for Treg cell development and function.32 Foxp3 mutations in the mice leads spontaneous development of allergic airway inflammation, hyper IgE syndrome, eosinophilia as well as autoimmune disease.26 Mutations in Foxp3 gene in humans leads formation of X-linked immune dysregulation polyendocrinopathy enteropathy syndrome (IPEX), hyper IgE syndrome and eczema.26 A dysregulation of disease-causing effector T cells is observed in atopic dermatitis lesions, in association with an impaired CD4+CD25+FoxP3+T-cell infiltration, despite the expression of type 1 regulatory cells in the dermis.33 Apart from these, main subsets of Treg cells, several other T cells with regulatory function has been described. Among them, suppressor capacity in vitro revealed CD8+ CD28- T cells, which are able to prevent up-regulation of B7 molecules induced by Th cells on professional APCs34 and play role in oral tolerance.35 TCRαβ+CD4-CD8- double-negative Treg cells have been shown to suppress Ag-specific immune responses mediated by CD8+ and CD4+ T cells in humans and mice.36 NKreg cell also has the capacity to suppress antigen specific T cell response.37 It has recently been demonstrated that, the transforming growth factor-beta (TGF-beta) induced the expression of the Runt-related transcription factors RUNX1 and RUNX3 in CD4+ T cells.6 This induction seems to be a prerequisite for the binding of RUNX1 and RUNX3 to three putative RUNX binding sites in the FOXP3 promoter. Further, in this study it was shown that RUNX transcription factors act as a molecular link in TGF-beta-induced Foxp3 expression in inducible Treg cell differentiation and function.
Tr1 cells are dominant type of T cell subset in healthy individuals. Studies clearly show that, allergen-specific Tr1 cells are predominant in healthy individuals to prevent unwanted immune response to nonpathogenic environmental antigens such as house dust mite, birch pollen, bee venom and food antigens (hazelnut, pear) which lead to allergy.38,39 Healthy and allergic individuals denote three different allergen-specific T cell subtypes as Th1, Th2 and Tr1 in different ratios.39 The imbalance between Th2 and Tr1 cells and depending the dominant subset may conduce allergy development or recovery from allergy. Peripheral T cell tolerance to venom allergen is an appropriate model for high dose tolerance to allergens in humans. During beekeeping season repeated exposure of non-allergic healthy beekeepers to bee venom antigens denote an efficient model to apprehend mechanisms of immune tolerance to bee venom allergens.40 During the exposure to venom allergen, venom specific IL-10-secreting Tr1 cells show a switch from allergen-specific Th1 and Th2 cells. This leads to suppression of allergen-specific undesired immune response by Th1 and Th2 cells. This immunomodulator response persist as long as venom exposure continuous and returns to the initial level within 2-3 months after the end of the beekeeping season and stimulation of histamine receptor 2 on Th2 cells by histamine suppresses allergen-stimulated T cells and enhance IL-10 production as an additional immune tolerance mechanism. Supporting these findings, non-allergic beekeepers have approximately 1,000 times higher allergen-specific IgG4 versus allergen-specific IgE ratio compared to bee venom allergic individuals.41 Another tolerance model with cat allergen also showed elevated levels of allergen-specific IgG4 levels after exposure to high dose cat allergen.42
With the knowledge of suppressive and immunomodulatory capacity of inducible or constitutive Treg cells, novel treatment strategies for T-cell mediated disease such as transplantation rejection, autoimmunity and allergy are being developed. The curative and preventive effect on disease conditions experienced by both adaptive transfer of regulatory T cells or their induction by immunomodulators in vivo are under consideration. Compared to conventional treatment strategies, their antigen-specific suppressor capacity as well as long-lasting antigen-specific regulation in vivo with a limited side effects were reported.43 Recent studies on immunomodulators that is targeting to enhance or suppress the numbers and functions of Treg cells, are rapamycin,44 co-stimulatory blockage,45 non-mitogenic anti-CD3 mAbs,46 T cell depletion47 and anti-TNF-α mAb48 (Table).49-75
Specifically expanded Treg cells can be targeted to allergen or an autoantigen expressed in the inflamed organs in murine models.76 Further, the transfer of this organ specific Treg cells can suppress an ongoing disease.77 These studies aimed for a successful therapeutical approach by targeting the Treg cell arm of immune tolerance against allergens, autoantigens or transplantation antigens. Several investigations are ongoing for adoptive transfer of Treg cells or small compounds aimed to induce Treg cells in the tissues.76 Other therapeutical approaches such as allergen-SIT, treatment with glucocorticoids, and beta-2 agonists seem to particially function by promoting the numbers and activity of IL-10-secreting Tr1-like cells.78-80
Allergen-specific immunotherapy, repeated administration of the sensitizing allergens by subcutaneous injection or mucosal route, has been used nearly 100 years ago by Noon and Freeman to grass polen allergic patiens with grass polen extracts. Clinical and experimental studies clearly show that SLIT is relatively safe method compared to subcutaneous immunotheraphy (SCIT) for the treatment of allergic disease,81,82 however the mechanisms of SLIT are less understood and its efficacy seems to be less than SCIT.83-85 Oral mucosal tissue has a natural tolerogenic character without any acute inflamation in spite of high bacterial colonization and good wound healing without scar development. Lack of inflammatory cells around mucosal tissue and high permeability for allergens suggests a way of action for sublingual allergen immunotherapy.86 The first step of SLIT is to uptake an allergen by Langerhans cells-specialized dendritic cells24 within the oral mucosa via high affinity surface IgE receptors.87 This leads to secretion of IL-10 and induction of T cells with a regulatory phenotype in vitro.88 Although clinical trails demonstrate treatment efficacy of SCIT in allergic asthma, allergic rhinitis, and stinging insect hypersensitivity as well as aero-allergen-induced atopic dermatitis such as reduction of allergic symptoms and drug intake, there is a risk of serious adverse reactions which can be classified in two categories: local reactions, which can appear as erythema, pruritus and swelling at the injection site; and systemic reactions can range in severity from mild to very severe life-threatening anaphylaxis.89-91
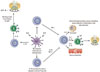
The primary purpose of allergen-SIT is the induction of peripheral T cell tolerance to allergens.28-31,39,92 Once peripheral T cell tolerance is triggered, allergen-specific Treg cells produce elaveted levels of IL-10 and TGF-β which are anti-inflammatory cytokines.29-31,38 The cytokines secreted from Treg cells mostly depend on the type of organ they dwell and the path in which they are stimulated. Experimental and clinical data revealed that Treg cells may secrete only IL-10, IL-10, and IFN-γ or IL-10 and TGF-β.25,39,93-96 Treg cells do not only suppress Th2 immune response and peripheral tolerance is achieved with multiple mechanism to overcome and suppress allergic inflammation. The other roles of Treg cells are suppression of dendritic cells and by this, enhance the generation of effector or induction of dendritic cells that support the generation of Treg cells,97-99 suppression of Th1 and Th2 cells,100 suppression of allergen-specific IgE and induction of IgG4 and/or IgA,101 suppression of mast cells, basophils and eosinophils,102 interaction with resident cells and remodelling.103,104 In SCIT both circulating30 and mucosal Tregs105 form and these Tregs may induce B cells to produce protective IgG4 antibodies106 and IgA2 antibodies.107 Proliferative response of T cell clones are also inhibited by IgG4 antibodies through prevention of IgE-faciliated allergen binding to B cells and subsequent presentation to allergen-specific T cell clones108 (Figure).
Mucosal immunotherapy to house dust mite, birch polen or food antigens leads to induction of Tr1 cells, which secrete IL-10 and TGF-β.31 Treg cells stimulated with toll-like receptors produce IL-10 and IFN-γ.94 After venom allergen-SIT, there is a induction of Tr1 cells producing only IL-10.109 Overall, it is obvious that allergen-SIT has a modulatory effect on allergen-specific T cells. In the mechanism of succesful allergen-SIT, shift in Th2 immunity to Th1 immune response is observed in peripheral blood,110,111 allergic rinitis112 and cutaneous late phase responses.110 One of the studies carried on patients with allergic rihinitis revealed that after grass pollen immunotherapy, Foxp3+ CD25+ and Foxp3+CD4+ cells numbers were found to be increased in the nasal mucosa.105 Also after this allergen-SIT, IL-10-producing Tr1 cells increased with supporting the role of Treg cells in the induction of allergen-specific tolerance in the humans.105 A very recent study showed that SIT with grass pollen extract leads to an increase in Foxp3+ cells in the sublingual epithelium.113
B cell activation and antibody production is an essential event during an immune response to antigens. Mature B cells show antibody class-switching in response to antigenic stimulation and costimulatory signals.114 Isotype class-switching of B cells depends mainly on cytokines released from T helper cells. In allergic immune response, Th2 cells secrete high amount of IL-4 and IL-13, which lead to ε-chain switching of B cells and the secretion of elevated levels of IgE. Other factors affecting the isotype swithing are CD40 and/or TLR stimulation. Even though peripheral T cell tolerance is rapidly induced after SIT, whether B cells tolerance takes place is still uncertain. Clinical data revealed that normal immune response to allergen results in increased production of allergen-specific IgE together with allergen-specific IgG4. In allergen-SIT, a transient increase in serum specific IgE115 is followed by gradual decrease over months or years of treatment. In addition, allergen-specific IgG4 and IgG1 subtypes of IgG antibody increase 10 to 100-fold in serum levels with successful allergen-SIT.49 Secreted IgG4 is thought to block the allergen before its binding and cross-linking of the IgE on the surface FcεRI receptors of mast cells and basophils, and by this inhibits activation and release of mediators responsible for type I hypersensitivity. However, there is poor correlation between the amount of allergen-specific IgG and clinical protection. Consequently, it becomes important to measure the blocking activity of allergen-specific IgG4 as well as IgG1 instead of their serum levels. In a clinical study of SLIT with grass polen extract, it was shown that IgG1 and IgG4 levels as well as seasonal IgA1 and IgA2 levels are increased.113 Also in this study, there is significant inhibition IgE-faciliated allergen binding to B cells at peak season, and time dependent increase in serum inhibitory activity for IgE-FAb in SLIT-treated atopic individuals.
IL-10 secreted from regulatory cells has a sophisticated role on the immune response with downregulation of T cells and induction of allergen-specific IgG4 antibodies. IL-10 counter-regulates allergen-specific IgE vs IgG4 levels.28,101 Thus, IL-10 regulates tolerance in T cells as well as inducing the IgG4 isotype rather than IgE phenotype. Clinical studies manifest during allergen SIT to sensitized patients, although there is no significant change in specific IgE levels, there is significant increase in specific IgA, IgG1, and IgG4 levels.31 Further studies also showed the correlation between increase of IgA and TGF-β and between increase of IgG4 and IL-10 in peripheral mucosal response to allergens in healthy individuals as well as patients treated with allergen-SIT.31,40 During allergen-SIT IgE/IgG4 ratio decreases and this is associated with a change from allergen-specific Th2 to Treg cell predominance. The change in IgE/IgG4 ratio is observed almost after several weeks due to early increase in IgG4.28 This is probably due to long living IgE plasma cells in the bone marrow. Significant decrease in IgE levels occurs in years in spite of early generation of Treg cells. Overall, allergen-SIT has immunomodulatory effect on antigen-specific T cells and B cells, and leads to early remission of clinical and latephase responses with a switch towards IgG4 in allergen-specific antibody levels.
In this review, we discussed the recent developments and more established knowledge on mechanisms of allergen-SIT. Peripheral T-cell tolerance is the key immunologic mechanism in the healthy immune response to self and non-infectious, non-self antigens. Induction of peripheral T cell tolerance by Treg cells is the main event that takes place in successful allergen-SIT. It is characterized by induction of Treg cells, suppressive cytokines such as IL-10 and also non-inflammatory antibody isotypes including IgG4 and IgA are essential to overcome allergic state. Knowledge of this molecular basis is pivotal in understanding the equilibrated regulation of the immune response and unresponsiveness to immunologic agents and their possible therapeutic applications. A crucial area for future studies is the identification of drugs, cytokines, or costimulatory molecules that induce peripheral T cell tolerance to environmental allergens. Novel vaccines that shorten the duration, decrease side effects, increase efficiency for treatment as well as novel preventive approach are expected due better understanding the mechanism of immune tolerance.
Figures and Tables
Figure
Mechanisms of allergen-specific immunotherapy. Both subcutaneous and sublingual SITs first affect the regional antigen-presenting cell, namely the local dendritic cell subset in the place of administration and draining lymph nodes. Although in vivo mechanisms are not clearly known, these dendritic cells induce Treg (CD4+CD25+FoxP3+) cells and Tr1 cells (IL-10+). Treg cells and regulatory cytokines (such as interleukin-10 (IL-10) and transforming growth factor-β, TGFβ) may contribute to the control of allergen-induced immune responses in several different ways. TReg cells utilize multiple suppressor factors to regulate the immune response. IL-10 and TGF-β suppress IgE production and IL-10 induces inflammatory immunoglobulin isotype, IgG4. These two cytokines directly suppress allergic inflammation induced by effector cells such as mast cells, basophils and eosinophils. TReg cells influence the generation of dendritic cells and promote the development of IL-10-producing dendritic cells. In addition, TReg cells inhibit Th2 cells, which can no longer provide cytokines such as IL-3, IL-4, IL-5, and IL-9. These cytokines are required for the differentiation, survival and activity of mast cells, basophils, eosinophils and mucus producing cells, as well as for the tissue homing of Th2 cells. SIT, specific immunotherapy; SLIT, sublingual immunotheraphy; SCIT, subcutaneous immunotheraphy; Treg, T regulatory cells.
References
1. Akdis CA. Allergy and hypersensitivity: mechanisms of allergic disease. Curr Opin Immunol. 2006. 18:718–726.
2. Palomares O, Yaman G, Azkur AK, Akkoc T, Akdis M, Akdis CA. Role of Treg in immune regulation of allergic diseases. Eur J Immunol. 2010. 40:1232–1240.
3. Akdis M, Akdis CA. Therapeutic manipulation of immune tolerance in allergic disease. Nat Rev Drug Discov. 2009. 8:645–660.
4. Simons FE. Anaphylaxis. J Allergy Clin Immunol. 2010. 125:S161–S181.
5. Kalesnikoff J, Galli SJ. New developments in mast cell biology. Nat Immunol. 2008. 9:1215–1223.
6. Basinski TM, Holzmann D, Eiwegger T, Zimmermann M, Klunker S, Meyer N, Schmid-Grendelmeier P, Jutel M, Akdis CA. Dual nature of T cell-epithelium interaction in chronic rhinosinusitis. J Allergy Clin Immunol. 2009. 124:74–80.e8.
7. Trautmann A, Akdis M, Kleemann D, Altznauer F, Simon HU, Graeve T, Noll M, Bröcker EB, Blaser K, Akdis CA. T cell-mediated Fas-induced keratinocyte apoptosis plays a key pathogenetic role in eczematous dermatitis. J Clin Invest. 2000. 106:25–35.
8. Sallusto F, Lanzavecchia A. Heterogeneity of CD4+ memory T cells: functional modules for tailored immunity. Eur J Immunol. 2009. 39:2076–2082.
9. Schmidt-Weber CB, Akdis M, Akdis CA. TH17 cells in the big picture of immunology. J Allergy Clin Immunol. 2007. 120:247–254.
10. Burgler S, Ouaked N, Bassin C, Basinski TM, Mantel PY, Siegmund K, Meyer N, Akdis CA, Schmidt-Weber CB. Differentiation and functional analysis of human T(H)17 cells. J Allergy Clin Immunol. 2009. 123:588–595.e7.
11. Truyen E, Coteur L, Dilissen E, Overbergh L, Dupont LJ, Ceuppens JL, Bullens DM. Evaluation of airway inflammation by quantitative Th1/Th2 cytokine mRNA measurement in sputum of asthma patients. Thorax. 2006. 61:202–208.
12. Schnyder-Candrian S, Togbe D, Couillin I, Mercier I, Brombacher F, Quesniaux V, Fossiez F, Ryffel B, Schnyder B. Interleukin-17 is a negative regulator of established allergic asthma. J Exp Med. 2006. 203:2715–2725.
13. Burgler S, Mantel PY, Bassin C, Ouaked N, Akdis CA, Schmidt-Weber CB. RORC2 is involved in T cell polarization through interaction with the FOXP3 promoter. J Immunol. 2010. 184:6161–6169.
14. Hellings PW, Kasran A, Liu Z, Vandekerckhove P, Wuyts A, Overbergh L, Mathieu C, Ceuppens JL. Interleukin-17 orchestrates the granulocyte influx into airways after allergen inhalation in a mouse model of allergic asthma. Am J Respir Cell Mol Biol. 2003. 28:42–50.
15. Ciprandi G, Filaci G, Battaglia F, Fenoglio D. Peripheral Th-17 cells in allergic rhinitis: new evidence. Int Immunopharmacol. 2010. 10:226–229.
16. Oboki K, Ohno T, Saito H, Nakae S. Th17 and allergy. Allergol Int. 2008. 57:121–134.
17. Eyerich S, Eyerich K, Pennino D, Carbone T, Nasorri F, Pallotta S, Cianfarani F, Odorisio T, Traidl-Hoffmann C, Behrendt H, Durham SR, Schmidt-Weber CB, Cavani A. Th22 cells represent a distinct human T cell subset involved in epidermal immunity and remodeling. J Clin Invest. 2009. 119:3573–3585.
18. Aujla SJ, Chan YR, Zheng M, Fei M, Askew DJ, Pociask DA, Reinhart TA, McAllister F, Edeal J, Gaus K, Husain S, Kreindler JL, Dubin PJ, Pilewski JM, Myerburg MM, Mason CA, Iwakura Y, Kolls JK. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat Med. 2008. 14:275–281.
19. Andoh A, Zhang Z, Inatomi O, Fujino S, Deguchi Y, Araki Y, Tsujikawa T, Kitoh K, Kim-Mitsuyama S, Takayanagi A, Shimizu N, Fujiyama Y. Interleukin-22, a member of the IL-10 subfamily, induces inflammatory responses in colonic subepithelial myofibroblasts. Gastroenterology. 2005. 129:969–984.
20. Colonna M, Trinchieri G, Liu YJ. Plasmacytoid dendritic cells in immunity. Nat Immunol. 2004. 5:1219–1226.
21. Lande R, Gilliet M. Plasmacytoid dendritic cells: key players in the initiation and regulation of immune responses. Ann N Y Acad Sci. 2010. 1183:89–103.
22. Lutz MB, Schuler G. Immature, semi-mature and fully mature dendritic cells: which signals induce tolerance or immunity? Trends Immunol. 2002. 23:445–449.
23. Fallarino F, Asselin-Paturel C, Vacca C, Bianchi R, Gizzi S, Fioretti MC, Trinchieri G, Grohmann U, Puccetti P. Murine plasmacytoid dendritic cells initiate the immunosuppressive pathway of tryptophan catabolism in response to CD200 receptor engagement. J Immunol. 2004. 173:3748–3754.
24. Allam JP, Stojanovski G, Friedrichs N, Peng W, Bieber T, Wenzel J, Novak N. Distribution of Langerhans cells and mast cells within the human oral mucosa: new application sites of allergens in sublingual immunotherapy? Allergy. 2008. 63:720–727.
25. Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008. 133:775–787.
26. Chatila TA. Role of regulatory T cells in human diseases. J Allergy Clin Immunol. 2005. 116:949–959. quiz 60.
27. Akdis M, Blaser K, Akdis CA. T regulatory cells in allergy: novel concepts in the pathogenesis, prevention, and treatment of allergic diseases. J Allergy Clin Immunol. 2005. 116:961–968. quiz 9.
28. Akdis CA, Akdis M, Blesken T, Wymann D, Alkan SS, Müller U, Blaser K. Epitope-specific T cell tolerance to phospholipase A2 in bee venom immunotherapy and recovery by IL-2 and IL-15 in vitro. J Clin Invest. 1996. 98:1676–1683.
29. Akdis CA, Blesken T, Akdis M, Wüthrich B, Blaser K. Role of interleukin 10 in specific immunotherapy. J Clin Invest. 1998. 102:98–106.
30. Francis JN, Till SJ, Durham SR. Induction of IL-10+CD4+CD25+ T cells by grass pollen immunotherapy. J Allergy Clin Immunol. 2003. 111:1255–1261.
31. Jutel M, Akdis M, Budak F, Aebischer-Casaulta C, Wrzyszcz M, Blaser K, Akdis CA. IL-10 and TGF-beta cooperate in the regulatory T-cell response to mucosal allergens in normal immunity and specific immunotherapy. Eur J Immunol. 2003. 33:1205–1214.
32. Klunker S, Chong MM, Mantel PY, Palomares O, Bassin C, Ziegler M, Rückert B, Meiler F, Akdis M, Littman DR, Akdis CA. Transcription factors RUNX1 and RUNX3 in the induction and suppressive function of Foxp3+ inducible regulatory T cells. J Exp Med. 2009. 206:2701–2715.
33. Verhagen J, Akdis M, Traidl-Hoffmann C, Schmid-Grendelmeier P, Hijnen D, Knol EF, Behrendt H, Blaser K, Akdis CA. Absence of T-regulatory cell expression and function in atopic dermatitis skin. J Allergy Clin Immunol. 2006. 117:176–183.
34. Zhou J, Appleton SE, Stadnyk A, Lee TD, Nashan BA. CD8+ gammadelta T regulatory cells mediate kidney allograft prolongation after oral exposure to alloantigen. Transpl Int. 2008. 21:679–687.
35. Ke Y, Kapp JA. Oral antigen inhibits priming of CD8+ CTL, CD4+ T cells, and antibody responses while activating CD8+ suppressor T cells. J Immunol. 1996. 156:916–921.
36. Chen W, Zhou D, Torrealba JR, Waddell TK, Grant D, Zhang L. Donor lymphocyte infusion induces long-term donor-specific cardiac xenograft survival through activation of recipient double-negative regulatory T cells. J Immunol. 2005. 175:3409–3416.
37. Deniz G, Erten G, Kücüksezer UC, Kocacik D, Karagiannidis C, Aktas E, Akdis CA, Akdis M. Regulatory NK cells suppress antigen-specific T cell responses. J Immunol. 2008. 180:850–857.
38. Akdis M. Healthy immune response to allergens: T regulatory cells and more. Curr Opin Immunol. 2006. 18:738–744.
39. Akdis M, Verhagen J, Taylor A, Karamloo F, Karagiannidis C, Crameri R, Thunberg S, Deniz G, Valenta R, Fiebig H, Kegel C, Disch R, Schmidt-Weber CB, Blaser K, Akdis CA. Immune responses in healthy and allergic individuals are characterized by a fine balance between allergen-specific T regulatory 1 and T helper 2 cells. J Exp Med. 2004. 199:1567–1575.
40. Meiler F, Zumkehr J, Klunker S, Ruckert B, Akdis CA, Akdis M. In vivo switch to IL-10-secreting T regulatory cells in high dose allergen exposure. J Exp Med. 2008. 205:2887–2898.
41. Carballido JM, Carballido-Perrig N, Kägi MK, Meloen RH, Wüthrich B, Heusser CH, Blaser K. T cell epitope specificity in human allergic and nonallergic subjects to bee venom phospholipase A2. J Immunol. 1993. 150:3582–3591.
42. Platts-Mills T, Vaughan J, Squillace S, Woodfolk J, Sporik R. Sensitisation, asthma, and a modified Th2 response in children exposed to cat allergen: a population-based cross-sectional study. Lancet. 2001. 357:752–756.
43. Frew AJ. Allergen immunotherapy. J Allergy Clin Immunol. 2010. 125:S306–S313.
44. Hendrikx TK, Velthuis JH, Klepper M, van Gurp E, Geel A, Schoordijk W, Baan CC, Weimar W. Monotherapy rapamycin allows an increase of CD4 CD25 FoxP3 T cells in renal recipients. Transpl Int. 2009. 22:884–891.
45. Kremer JM, Dougados M, Emery P, Durez P, Sibilia J, Shergy W, Steinfeld S, Tindall E, Becker JC, Li T, Nuamah IF, Aranda R, Moreland LW. Treatment of rheumatoid arthritis with the selective costimulation modulator abatacept: twelve-month results of a phase iib, double-blind, randomized, placebo-controlled trial. Arthritis Rheum. 2005. 52:2263–2271.
46. Utset TO, Auger JA, Peace D, Zivin RA, Xu D, Jolliffe L, Alegre ML, Bluestone JA, Clark MR. Modified anti-CD3 therapy in psoriatic arthritis: a phase I/II clinical trial. J Rheumatol. 2002. 29:1907–1913.
47. Isaacs JD, Greer S, Sharma S, Symmons D, Smith M, Johnston J, Waldmann H, Hale G, Hazleman BL. Morbidity and mortality in rheumatoid arthritis patients with prolonged and profound therapy-induced lymphopenia. Arthritis Rheum. 2001. 44:1998–2008.
48. Ehrenstein MR, Evans JG, Singh A, Moore S, Warnes G, Isenberg DA, Mauri C. Compromised function of regulatory T cells in rheumatoid arthritis and reversal by anti-TNFalpha therapy. J Exp Med. 2004. 200:277–285.
49. Jutel M, Jaeger L, Suck R, Meyer H, Fiebig H, Cromwell O. Allergen-specific immunotherapy with recombinant grass pollen allergens. J Allergy Clin Immunol. 2005. 116:608–613.
50. Kussebi F, Karamloo F, Rhyner C, Schmid-Grendelmeier P, Salagianni M, Mannhart C, Akdis M, Soldatova L, Markovic-Housley Z, Von Beust BR, Kundig T, Kemeny DM, Blaser K, Crameri R, Akdis CA. A major allergen gene-fusion protein for potential usage in allergen-specific immunotherapy. J Allergy Clin Immunol. 2005. 115:323–329.
51. Karamloo F, Schmid-Grendelmeier P, Kussebi F, Akdis M, Salagianni M, von Beust BR, Reimers A, Zumkehr J, Soldatova L, Housley-Markovic Z, Müller U, Kündig T, Kemeny DM, Spangfort MD, Blaser K, Akdis CA. Prevention of allergy by a recombinant multi-allergen vaccine with reduced IgE binding and preserved T cell epitopes. Eur J Immunol. 2005. 35:3268–3276.
52. Niederberger V, Horak F, Vrtala S, Spitzauer S, Krauth MT, Valent P, Reisinger J, Pelzmann M, Hayek B, Kronqvist M, Gafvelin G, Gronlund H, Purohit A, Suck R, Fiebig H, Cromwell O, Pauli G, van Hage-Hamsten M, Valenta R. Vaccination with genetically engineered allergens prevents progression of allergic disease. Proc Natl Acad Sci U S A. 2004. 101:Suppl 2. 14677–14682.
53. Larché M. Immunoregulation by targeting T cells in the treatment of allergy and asthma. Curr Opin Immunol. 2006. 18:745–750.
54. Alexander C, Tarzi M, Larché M, Kay AB. The effect of Fel d 1-derived T-cell peptides on upper and lower airway outcome measurements in cat-allergic subjects. Allergy. 2005. 60:1269–1274.
55. Norman PS, Ohman JL Jr, Long AA, Creticos PS, Gefter MA, Shaked Z, Wood RA, Eggleston PA, Hafner KB, Rao P, Lichtenstein LM, Jones NH, Nicodemus CF. Treatment of cat allergy with T-cell reactive peptides. Am J Respir Crit Care Med. 1996. 154:1623–1628.
56. Müller U, Akdis CA, Fricker M, Akdis M, Blesken T, Bettens F, Blaser K. Successful immunotherapy with T-cell epitope peptides of bee venom phospholipase A2 induces specific T-cell anergy in patients allergic to bee venom. J Allergy Clin Immunol. 1998. 101:747–754.
57. Marcotte GV, Braun CM, Norman PS, Nicodemus CF, Kagey-Sobotka A, Lichtenstein LM, Essayan DM. Effects of peptide therapy on ex vivo T-cell responses. J Allergy Clin Immunol. 1998. 101:506–513.
58. von Garnier C, Astori M, Kettner A, Dufour N, Heusser C, Corradin G, Spertini F. Allergen-derived long peptide immunotherapy downregulates specific IgE response and protects from anaphylaxis. Eur J Immunol. 2000. 30:1638–1645.
59. Haselden BM, Kay AB, Larché M. Immunoglobulin E-independent major histocompatibility complex-restricted T cell peptide epitope-induced late asthmatic reactions. J Exp Med. 1999. 189:1885–1894.
60. Oldfield WL, Larché M, Kay AB. Effect of T-cell peptides derived from Fel d 1 on allergic reactions and cytokine production in patients sensitive to cats: a randomised controlled trial. Lancet. 2002. 360:47–53.
61. Akdis CA, Blaser K. Bypassing IgE and targeting T cells for specific immunotherapy of allergy. Trends Immunol. 2001. 22:175–178.
62. Kahlert H, Suck R, Weber B, Nandy A, Wald M, Keller W, Cromwell O, Fiebig H. Characterization of a hypoallergenic recombinant Bet v 1 variant as a candidate for allergen-specific immunotherapy. Int Arch Allergy Immunol. 2008. 145:193–206.
63. Pree I, Reisinger J, Focke M, Vrtala S, Pauli G, van Hage M, Cromwell O, Gadermaier E, Egger C, Reider N, Horak F, Valenta R, Niederberger V. Analysis of epitope-specific immune responses induced by vaccination with structurally folded and unfolded recombinant Bet v 1 allergen derivatives in man. J Immunol. 2007. 179:5309–5316.
64. Creticos PS, Schroeder JT, Hamilton RG, Balcer-Whaley SL, Khattignavong AP, Lindblad R, Li H, Coffman R, Seyfert V, Eiden JJ, Broide D. Immunotherapy with a ragweed-toll-like receptor 9 agonist vaccine for allergic rhinitis. N Engl J Med. 2006. 355:1445–1455.
65. Patel P, Salapatek AM. Pollinex Quattro: a novel and well-tolerated, ultra short-course allergy vaccine. Expert Rev Vaccines. 2006. 5:617–629.
66. Puggioni F, Durham SR, Francis JN. Monophosphoryl lipid A (MPL) promotes allergen-induced immune deviation in favour of Th1 responses. Allergy. 2005. 60:678–684.
67. Assessment of the contribution of monophosphoryl lipid A (MPL) to a grass pollen allergy vaccine [Internet]. U.S. National Institute of Health. updated 2010 Jun 16. Available from: http://clinicaltrials.gov/ct2/show/NCT00133146.
68. Kundig TM, Senti G, Schnetzler G, Wolf C, Prinz Vavricka BM, Fulurija A, Hennecke F, Sladko K, Jennings GT, Bachmann MF. Der p 1 peptide on virus-like particles is safe and highly immunogenic in healthy adults. J Allergy Clin Immunol. 2006. 117:1470–1476.
69. Casale TB, Busse WW, Kline JN, Ballas ZK, Moss MH, Townley RG, Mokhtarani M, Seyfert-Margolis V, Asare A, Bateman K, Deniz Y. Omalizumab pretreatment decreases acute reactions after rush immunotherapy for ragweed-induced seasonal allergic rhinitis. J Allergy Clin Immunol. 2006. 117:134–140.
70. Johansen P, Häffner AC, Koch F, Zepter K, Erdmann I, Maloy K, Simard JJ, Storni T, Senti G, Bot A, Wüthrich B, Kündig TM. Direct intralymphatic injection of peptide vaccines enhances immunogenicity. Eur J Immunol. 2005. 35:568–574.
71. Senti G, Prinz Vavricka BM, Erdmann I, Diaz MI, Markus R, Mc-Cormack SJ, Simard JJ, Wüthrich B, Crameri R, Graf N, Johansen P, Kündig TM. Intralymphatic allergen administration renders specific immunotherapy faster and safer: a randomized controlled trial. Proc Natl Acad Sci U S A. 2008. 105:17908–17912.
72. Zhu D, Kepley CL, Zhang M, Zhang K, Saxon A. A novel human immunoglobulin Fc gamma Fc epsilon bifunctional fusion protein inhibits Fc epsilon RI-mediated degranulation. Nat Med. 2002. 8:518–521.
73. Zhu D, Kepley CL, Zhang K, Terada T, Yamada T, Saxon A. A chimeric human-cat fusion protein blocks cat-induced allergy. Nat Med. 2005. 11:446–449.
74. Rhyner C, Kündig T, Akdis CA, Crameri R. Targeting the MHC II presentation pathway in allergy vaccine development. Biochem Soc Trans. 2007. 35:833–834.
75. Evaluation of safety, tolerability, immunogenicity and efficacy of a novel method in specific immunotherapy in cat allergic patients: a placebo controlled trial (IVN-CAT-001B) [Internet]. U.S. National Institute of Health. updated 2010 Feb 10. Available from: http://clinicaltrials.gov/ct2/show/study/NCT00718679.
76. O'Connor RA, Anderton SM. Multi-faceted control of autoaggression: Foxp3+ regulatory T cells in murine models of organ-specific autoimmune disease. Cell Immunol. 2008. 251:8–18.
77. Roncarolo MG, Battaglia M. Regulatory T-cell immunotherapy for tolerance to self antigens and alloantigens in humans. Nat Rev Immunol. 2007. 7:585–598.
78. Peek EJ, Richards DF, Faith A, Lavender P, Lee TH, Corrigan CJ, Hawrylowicz CM. Interleukin-10-secreting "regulatory" T cells induced by glucocorticoids and beta2-agonists. Am J Respir Cell Mol Biol. 2005. 33:105–111.
79. Karagiannidis C, Akdis M, Holopainen P, Woolley NJ, Hense G, Ruckert B, Mantel PY, Menz G, Akdis CA, Blaser K, Schmidt-Weber CB. Glucocorticoids upregulate FOXP3 expression and regulatory T cells in asthma. J Allergy Clin Immunol. 2004. 114:1425–1433.
80. Akdis M, Akdis CA. Mechanisms of allergen-specific immunotherapy. J Allergy Clin Immunol. 2007. 119:780–791.
81. Wilson DR, Lima MT, Durham SR. Sublingual immunotherapy for allergic rhinitis: systematic review and meta-analysis. Allergy. 2005. 60:4–12.
82. Gidaro GB, Marcucci F, Sensi L, Incorvaia C, Frati F, Ciprandi G. The safety of sublingual-swallow immunotherapy: an analysis of published studies. Clin Exp Allergy. 2005. 35:565–571.
83. Calderon MA, Alves B, Jacobson M, Hurwitz B, Sheikh A, Durham S. Allergen injection immunotherapy for seasonal allergic rhinitis. Cochrane Database Syst Rev. 2007. CD001936.
84. Calderón MA, Penagos M, Durham SR. Sublingual immunotherapy for allergic Rhinoconjunctivitis, allergic asthma, and prevention of allergic diseases. Clin Allergy Immunol. 2008. 21:359–375.
85. Novak N, Haberstok J, Bieber T, Allam JP. The immune privilege of the oral mucosa. Trends Mol Med. 2008. 14:191–198.
86. Marcucci F, Incorvaia C, Sensi L, Di Cara G, Cadario G, Cavaliere A, Moingeon P, Puccinelli P, Di Gioacchino M, Frati F. Lack of inflammatory cells in the oral mucosa of subjects undergoing sublingual immunotherapy. Int J Immunopathol Pharmacol. 2008. 21:609–613.
87. Allam JP, Novak N, Fuchs C, Asen S, Bergé S, Appel T, Geiger E, Kochan JP, Bieber T. Characterization of dendritic cells from human oral mucosa: a new Langerhans' cell type with high constitutive FcepsilonRI expression. J Allergy Clin Immunol. 2003. 112:141–148.
88. Allam JP, Peng WM, Appel T, Wenghoefer M, Niederhagen B, Bieber T, Bergé S, Novak N. Toll-like receptor 4 ligation enforces tolerogenic properties of oral mucosal Langerhans cells. J Allergy Clin Immunol. 2008. 121:368–374.e1.
89. Cox L, Larenas-Linnemann D, Lockey RF, Passalacqua G. Speaking the same language: The World Allergy Organization Subcutaneous Immunotherapy Systemic Reaction Grading System. J Allergy Clin Immunol. 2010. 125:569–574.e7.
90. Werfel T, Breuer K, Ruéff F, Przybilla B, Worm M, Grewe M, Ruzicka T, Brehler R, Wolf H, Schnitker J, Kapp A. Usefulness of specific immunotherapy in patients with atopic dermatitis and allergic sensitization to house dust mites: a multi-centre, randomized, dose-response study. Allergy. 2006. 61:202–205.
91. Jacobsen L, Niggemann B, Dreborg S, Ferdousi HA, Halken S, Høst A, Koivikko A, Norberg LA, Valovirta E, Wahn U, Möller C. Specific immunotherapy has long-term preventive effect of seasonal and perennial asthma: 10-year follow-up on the PAT study. Allergy. 2007. 62:943–948.
92. Ling EM, Smith T, Nguyen XD, Pridgeon C, Dallman M, Arbery J, Carr VA, Robinson DS. Relation of CD4+CD25+ regulatory T-cell suppression of allergen-driven T-cell activation to atopic status and expression of allergic disease. Lancet. 2004. 363:608–615.
93. Groux H, O'Garra A, Bigler M, Rouleau M, Antonenko S, de Vries JE, Roncarolo MG. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997. 389:737–742.
94. Akdis CA, Kussebi F, Pulendran B, Akdis M, Lauener RP, Schmidt-Weber CB, Klunker S, Isitmangil G, Hansjee N, Wynn TA, Dillon S, Erb P, Baschang G, Blaser K, Alkan SS. Inhibition of T helper 2-type responses, IgE production and eosinophilia by synthetic lipopeptides. Eur J Immunol. 2003. 33:2717–2726.
95. Izcue A, Coombes JL, Powrie F. Regulatory lymphocytes and intestinal inflammation. Annu Rev Immunol. 2009. 27:313–338.
96. Izcue A, Hue S, Buonocore S, Arancibia-Cárcamo CV, Ahern PP, Iwakura Y, Maloy KJ, Powrie F. Interleukin-23 restrains regulatory T cell activity to drive T cell-dependent colitis. Immunity. 2008. 28:559–570.
97. Steinbrink K, Wölfl M, Jonuleit H, Knop J, Enk AH. Induction of tolerance by IL-10-treated dendritic cells. J Immunol. 1997. 159:4772–4780.
98. Wu K, Bi Y, Sun K, Xia J, Wang Y, Wang C. Suppression of allergic inflammation by allergen-DNA-modified dendritic cells depends on the induction of Foxp3+ Regulatory T cells. Scand J Immunol. 2008. 67:140–151.
99. Bellinghausen I, Brand U, Steinbrink K, Enk AH, Knop J, Saloga J. Inhibition of human allergic T-cell responses by IL-10-treated dendritic cells: differences from hydrocortisone-treated dendritic cells. J Allergy Clin Immunol. 2001. 108:242–249.
100. Kearley J, Barker JE, Robinson DS, Lloyd CM. Resolution of airway inflammation and hyperreactivity after in vivo transfer of CD4+CD25+ regulatory T cells is interleukin 10 dependent. J Exp Med. 2005. 202:1539–1547.
101. Meiler F, Klunker S, Zimmermann M, Akdis CA, Akdis M. Distinct regulation of IgE, IgG4 and IgA by T regulatory cells and toll-like receptors. Allergy. 2008. 63:1455–1463.
102. Gri G, Piconese S, Frossi B, Manfroi V, Merluzzi S, Tripodo C, Viola A, Odom S, Rivera J, Colombo MP, Pucillo CE. CD4+CD25+ regulatory T cells suppress mast cell degranulation and allergic responses through OX40-OX40L interaction. Immunity. 2008. 29:771–781.
103. Kearley J, Robinson DS, Lloyd CM. CD4+CD25+ regulatory T cells reverse established allergic airway inflammation and prevent airway remodeling. J Allergy Clin Immunol. 2008. 122:617–624.e6.
104. Burchell JT, Wikstrom ME, Stumbles PA, Sly PD, Turner DJ. Attenuation of allergen-induced airway hyperresponsiveness is mediated by airway regulatory T cells. Am J Physiol Lung Cell Mol Physiol. 2009. 296:L307–L319.
105. Radulovic S, Jacobson MR, Durham SR, Nouri-Aria KT. Grass pollen immunotherapy induces Foxp3-expressing CD4+CD25+ cells in the nasal mucosa. J Allergy Clin Immunol. 2008. 121:1467–1472.e1.
106. James LK, Durham SR. Update on mechanisms of allergen injection immunotherapy. Clin Exp Allergy. 2008. 38:1074–1088.
107. Pilette C, Nouri-Aria KT, Jacobson MR, Wilcock LK, Detry B, Walker SM, Francis JN, Durham SR. Grass pollen immunotherapy induces an allergen-specific IgA2 antibody response associated with mucosal TGF-beta expression. J Immunol. 2007. 178:4658–4666.
108. van Neerven RJ, Arvidsson M, Ipsen H, Sparholt SH, Rak S, Würtzen PA. A double-blind, placebo-controlled birch allergy vaccination study: inhibition of CD23-mediated serum-immunoglobulin E-facilitated allergen presentation. Clin Exp Allergy. 2004. 34:420–428.
109. Akdis CA, Blaser K. IL-10-induced anergy in peripheral T cell and reactivation by microenvironmental cytokines: two key steps in specific immunotherapy. FASEB J. 1999. 13:603–609.
110. Varney VA, Hamid QA, Gaga M, Ying S, Jacobson M, Frew AJ, Kay AB, Durham SR. Influence of grass pollen immunotherapy on cellular infiltration and cytokine mRNA expression during allergen-induced late-phase cutaneous responses. J Clin Invest. 1993. 92:644–651.
111. Jutel M, Pichler WJ, Skrbic D, Urwyler A, Dahinden C, Müller UR. Bee venom immunotherapy results in decrease of IL-4 and IL-5 and increase of IFN-gamma secretion in specific allergen-stimulated T cell cultures. J Immunol. 1995. 154:4187–4194.
112. Durham SR, Ying S, Varney VA, Jacobson MR, Sudderick RM, Mackay IS, Kay AB, Hamid QA. Grass pollen immunotherapy inhibits allergen-induced infiltration of CD4+ T lymphocytes and eosinophils in the nasal mucosa and increases the number of cells expressing messenger RNA for interferon-gamma. J Allergy Clin Immunol. 1996. 97:1356–1365.
113. Scadding GW, Shamji MH, Jacobson MR, Lee DI, Wilson D, Lima MT, Pitkin L, Pilette C, Nouri-Aria K, Durham SR. Sublingual grass pollen immunotherapy is associated with increases in sublingual Foxp3-expressing cells and elevated allergen-specific immunoglobulin G4, immunoglobulin A and serum inhibitory activity for immunoglobulin E-facilitated allergen binding to B cells. Clin Exp Allergy. 2010. 40:598–606.
114. Stavnezer J, Guikema JE, Schrader CE. Mechanism and regulation of class switch recombination. Annu Rev Immunol. 2008. 26:261–292.
115. Müller U, Helbling A, Bischof M. Predictive value of venom-specific IgE, IgG and IgG subclass antibodies in patients on immunotherapy with honey bee venom. Allergy. 1989. 44:412–418.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download