Abstract
Human toxocariasis is the most prevalent helminthiasis in Korea and other industrialized countries. The clinical features of toxocariasis are diverse, according to the involved organ. Typically, Toxocara spp. infection is easily treated with 400 mg albendazole twice a day for 5 days. However, we experienced a case of recurrent toxocariasis that was refractory to this standard therapy and presented with urticaria, an uncommon symptom in toxocariasis. A 35-year-old male visited our emergency room because of abdominal pain. He had recently consumed raw cow liver (3 weeks prior to presentation). Laboratory analyses revealed eosinophilia (1,612 cells/µL) and increased total IgE (3,060 IU/mL). Chest X-ray showed multiple lung nodules in both lungs, and computed tomography revealed multiple ground-glass opacities in both lungs and multiple tiny liver abscesses. Liver biopsy revealed an eosinophilic abscess. Enzyme-linked immunosorbent assay findings for Toxocara antigens were positive (optical density, 2.140), leading to a diagnosis of toxocariasis. We initiated a 5-day treatment with albendazole and prednisolone; however, 6 days after completing the treatment, the patient again experienced urticaria and severe itching that could not be controlled by antihistamines or hydrocortisone cream. A second bout of eosinophilia suggested recurring toxocariasis, for which we prescribed a second round of albendazole. Despite an initial improvement in his symptoms, the patient returned after 6 weeks complaining of abdominal pain for 6 hours, which was reminiscent of his first attack; he also exhibited eosinophilia. Accordingly, albendazole was administered once more for an additional 3 weeks, and his symptoms resolved.
Toxocariasis is a helminthozoonosis caused by ascarid larvae of the Toxocara genus.1 It is the most common helminthiasis in industrialized countries,1 including Korea.2 Human toxocariasis is caused by geophagia (pica), close contact with dogs or cats, poor personal hygiene, ingestion of contaminated raw vegetables, or ingestion of raw meat from a paratenic host.1,3,4 As the rate of pet ownership in Korea increases, a concomitant increase in the risk for contracting toxocariasis will require physicians to become familiar with the clinical features and treatment of this disease. Toxocara infection may present with various symptoms affecting different organs.1,3 Dermatologically, toxocariasis may be associated with pruritus, rash, and chronic urticaria, with very few reports of acute urticaria.1,3,5 Toxocariasis is typically treated easily with albendazole,1,3 and recurrent disease has not been reported in Korea. Here, we report a case of recurrent toxocariasis accompanied by abdominal pain and urticaria that was resistant to routine treatment.
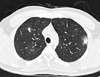
A 35-year-old male visited our emergency room because of cramping epigastric pain; he had no past medical history of this kind. Although the patient had previously consumed raw fish, his most recent ingestion of potentially contaminated food was raw cow liver, 3 weeks prior to presentation. The patient's blood pressure was 130/70 mmHg; his heart rate was 75/min; and his body temperature was 36℃. Blood analyses showed peripheral eosinophilia (leukocytes, 13.11×103/µL, with 58.6% neutrophils, 22.4% lymphocytes, 4.9% monocytes, and 12.3% eosinophils), an aspartate aminotransferase level of 19 U/L, and an alanine aminotransferase level of 17 U/L. The patient tested negative for HBsAg and anti-HCV antibody, and positive for anti-HBs antibody. Prothrombin time and activated partial thromboplastin time were normal. The patient's level of C-reactive protein was normal at 0.16 mg/dL (0-0.5 mg/dL). Urinalysis and stool examination revealed no abnormalities. The patient's serum total immunoglobulin (Ig) E level was 3,060 IU/mL, as determined using a paper radioimmunsorbent test. Physical examination revealed tenderness in the epigastric area. Although no abnormality was observed upon abdominal X-ray, chest X-ray revealed multiple nodules in both lungs, and abdominal and chest computed tomography revealed multiple tiny liver abscesses (Fig. 1) and multiple ground-glass opacities in both lungs (Fig. 2). Liver biopsy showed patchy, dense eosinophilic hepatic infiltrates, suggestive of eosinophilic abscess (Fig. 3). Bone marrow biopsy revealed normocellular marrow with increased eosinophils. Gastroscopy at the time of epigastric pain showed no evidence of other parasitic infections such as anisakiasis. When we measured Toxocara canis larva E/S antigen-specific IgG using an enzyme-linked immunosorbent assay kit (TES-ELISA; Bordier Affinity Products SA, Crissier, Switzerland), the observed optical density (2.140) suggested the presence of Toxocara larva at a relatively high titer when compared against the positive (0.616) and negative (0.033) controls. We then initiated treatment with 400 mg albendazole twice a day (the patient's weight was 80 kg) and 60 mg prednisolone for 5 days. However, 6 days after the cessation of albendazole, the patient again experienced generalized urticaria and itching that could not be controlled through the use of antihistamines or hydrocortisone cream. The possibility of a drug allergy was considered unlikely given the lapse in time between drug treatment and presentation of symptoms. Because the patient showed eosinophilia (increasing from 46 to 1,262 cells/µL) and newly developed urticaria that was refractory to conventional treatment, we suspected the recurrence or inadequate treatment of toxocariasis. A second round of 400 mg albendazole once daily for 2 weeks was initiated, and this eliminated both urticaria and itching within 1 day. The patient was discharged with improved symptoms after 1 week. Six weeks after discharge, the patient returned to the emergency room, again complaining of cramping epigastric pain reminiscent of his first attack of toxocariasis. Repeated gastroscopy revealed only chronic gastritis. The laboratory test showed an elevated eosinophil count (increasing from 543 to 1,335 cells/µL). We suspected recurrent toxocariasis and prescribed 400 mg albendazole once daily. After treatment, the patient's symptoms appeared to be resolved and his eosinophil count had normalized. After a 3-week treatment with albendazole, he has remained symptom-free with no further relapses for 2 years. His most recent eosinophil count was 188 cells/µL (Fig. 4).
Since the first report of human toxocariasis in the 1950s, toxocariasis has become the most prevalent helminthiasis in industrialized countries.1,3 In 2002, Park et al.2 reported that the seroprevalence of toxocariasis in Korean rural adults was approximately 5%, based on research in Gangwon province. After ingestion as embryonated eggs or adults, Toxocara can live in the small intestine of dogs or cats. Eggs escape the intestine through defecation and become embryonated while resting on or in the soil. Humans may ingest the eggs accidentally through contact with pet feces, eating contaminated raw vegetables, or consuming raw meat from a paratenic host.1,3 The patient described here most likely contracted toxocariasis as a result of consuming raw cow liver (not raw fish); it has been reported that a recent history of eating raw cow liver is related to increased risk for toxocariasis in patients with eosinophilia of unknown etiology in Korea.4
Although Toxocara eggs may hatch in the human small intestine, larvae cannot develop into adults in humans and thus remain in a juvenile stage.1,3 Larvae then penetrate the intestinal wall and migrate to multiple organs via the blood stream; diverse clinical features may develop according to the involved organ.1,3 Toxocariasis is classified as visceral larva migrans (VLM) when it involves the major organs, or ocular larva migrans when it involves the eye and optic nerve.1,3 The patient described here demonstrated the clinical features of VLM: abdominal pain, eosinophilia, pulmonary focal ground-glass opacities, and hepatic eosinophilic abscess. Immediate hypersensitivity to dying or dead larvae in organs may also elicit fever, enlargement of the liver or spleen, lower respiratory symptoms resembling asthma, hypergammaglobulinemia, myocarditis, nephritis, central nervous system involvement, and other conditions.1,3 In the present case, the patient complained of abdominal pain in the first attack, urticaria during the second attack, and abdominal pain again during the third attack.
Many studies have reported an association between chronic urticaria and toxocariasis,5,6 but few reports describe acute urticaria, particularly in association with recurrent disease.6 Although skin lesions are rarely the only symptom of toxocariasis, previous studies and the case described here demonstrate that urticaria may be a sign of toxocariasis or other parasitic infections.6 Although we initially considered the possibility of anisakiasis, we excluded this condition because the patient had not recently consumed raw fish and because gastroscopy showed no physical evidence of the parasite. Instead, the patient was diagnosed with toxocariasis based on the detection of T. canis E/S antigens at a high titer. However, given that immunodiagnostic tests cannot discriminate between current and past infections, it is important to determine the blood eosinophil count and serum total IgE together when recurrence is suspected.1
Recurrent disease was resolved through prolonged albendazole treatment. Albendazole is the treatment of choice for toxocariasis, with a currently recommended regimen of 10 mg/kg/day in two doses (400 mg twice daily) for 5 days.1,3 Interestingly, recent clinical studies have shown that relatively few patients, only 6 of 19 (32%), are cured after 5-day albendazole therapy.7,8 Instead, in Japan, adults who contract toxocariasis (typically through eating raw liver) are treated with albendazole for 4 weeks or longer.7,9 Accordingly, further study is required to reach a consensus regarding the appropriate duration of albendazole therapy to eliminate toxocariasis.
Figures and Tables
References
1. Magnaval JF, Glickman LT, Dorchies P, Morassin B. Highlights of human toxocariasis. Korean J Parasitol. 2001; 39:1–11.
2. Park HY, Lee SU, Huh S, Kong Y, Magnaval JF. A seroepidemiological survey for toxocariasis in apparently healthy residents in Gangwon-do, Korea. Korean J Parasitol. 2002; 40:113–117.
3. Despommier D. Toxocariasis: clinical aspects, epidemiology, medical ecology, and molecular aspects. Clin Microbiol Rev. 2003; 16:265–272.
4. Choi D, Lim JH, Choi DC, Paik SW, Kim SH, Huh S. Toxocariasis and ingestion of raw cow liver in patients with eosinophilia. Korean J Parasitol. 2008; 46:139–143.
5. Gavignet B, Piarroux R, Aubin F, Millon L, Humbert P. Cutaneous manifestations of human toxocariasis. J Am Acad Dermatol. 2008; 59:1031–1042.
6. Humbert P, Niezborala M, Salembier R, Aubin F, Piarroux R, Buchet S, Barale T. Skin manifestations associated with toxocariasis: a case-control study. Dermatology. 2000; 201:230–234.
7. Yoshikawa M. Duration of treatment with albendazole for hepatic toxocariasis. Nat Clin Pract Gastroenterol Hepatol. 2009; 6:E1–E2.
8. Stürchler D, Schubarth P, Gualzata M, Gottstein B, Oettli A. Thiabendazole vs. albendazole in treatment of toxocariasis: a clinical trial. Ann Trop Med Parasitol. 1989; 83:473–478.
9. Takamatsu K, Sumitani M, Nanjyou S, Nishijima M, Syoji S, Takifuji N, Kiyota H, Daga H, Takeda K. Case of Toxocara canis larva migrans cured by additional treatment with albendazole. Nihon Kokyuki Gakkai Zasshi. 2008; 46:836–841.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download