Abstract
Staphylococcus aureus (SA) is usually present not only in the skin lesions of atopic dermatitis (AD) but also in the atopic dry skin. SA discharges various toxins and enzymes that injure the skin, results in activation of epidermal keratinocytes, which produce and release IL-18. IL-18 that induces the super Th1 cells secreting IFN-γ and IL-13 is supposed to be involved in development of AD and its pathogenesis. Indeed, the number of SA colonies on the skin surface and the serum IL-18 levels in patients with AD significantly correlated with the skin scores of AD lesions. Also, there is strong positive correlation between the skin scores and serum IL-18 levels in DS-Nh mice (P<0.0001, r=0.64), which develop considerable AD-like legions when they are housed under conventional conditions, but develop skin legions with less severity and less frequency under specific pathogens free (SPF) conditions. Therefore, they are well-known as model mice of AD, in which SA is presumed to be critical factor for the development of AD lesions. Also, theses DS-Nh mice pretreated with Cy developed more remarkable AD-like lesions in comparison with non-treated ones. The levels of INF-r and IL-13 in the supernatants of the lymph node cell cultures stimulated with staphylococcal enterotoxin B (SEB) or ConA were increased in the Cy-treated mice, although the serum levels of total IgE were not. In this experiment, we revealed that Cy-treated mice, to which CD25 +CD4 + reguratory T cells taken from non-treated ones had been transferred, developed the AD-like legions with less severity and less number of SA colonies on the skin surface. Therefore, it is presumed that CD25 +CD4 + reguratory T cells might be involved in the suppression of super Th1 cells which are induced by IL-18 and are involved in the development of AD-like lesions rather than IgE production. The efficient induction of CD25 +CD4 + reguratory T cells is expected for the new type of treatment of AD. We also found that farnesol (F) and xylitol (X) synergistically inhibited biofilm formation by SA, and indeed the ratio of SA in total bacteria at sites to which the FX cream containing F and X had been applied was significantly decreased 1 week later, accompanied with improvement of AD, when compared with that before application and at placebo sites. Therefore, the FX cream is a useful skin-care agent for atopic dry skin colonized by SA. The nerve growth factor (NGF) in the horny layer (the horn NGF) of skin lesions on the cubital fossa was collected by tape stripping and measured using ELISA in AD patients before and after 2 and 4 weeks treatments. Simultaneously, the itch and eruptions on the whole body and on the lesions, in which the horn NGF was measured, were recorded, and also the peripheral blood eosinophil count, serum LDH level and serum total IgE level were examined. The level of NGF was significantly higher in AD patients than in healthy controls, correlated with the severity of itch, erythema, scale/xerosis, the eosinophil count and LDH level, and also significantly decreased after treatments with olopatadine and/or steroid ointment for 2 and 4 weeks. Therefore, the measurement of the NGF by this harmless method seems to be useful to assess the severity of AD and the therapeutic effects on AD. In AD patients, C-fiber in the epidermis increase and sprout, inducing hypersensitivity, which is considered to aggravate the disease. Semaphorin 3A (Sema3A), an axon guidance molecule, is a potent inhibitor of neurite outgrowth of sensory neurons. We administered recombinant Sema3A intracutaneously into the skin lesions of NC/Nga mice, an animal model of AD, and investigated the effect of Sema3A on the skin lesions and their itch. Sema3A dose-dependently improved skin lesions and attenuated the scratching behavior in NC/Nga mice. Histological examinations revealed a decrease in the epidermal thickness, the density of invasive nerve fibers in the epidermis, inflammatory infiltrate including mast cells and CD4 +T cells, and the production of IL-4 in the Sema3A-treated lesions. Because the interruption of the itch-scratch cycle likely contributes to the improvement of the AD-like lesions, Sema3A is expected to become a promising treatment of patients with refractory AD.
Atopic dermatitis (AD) is a chronic, relapsing, inflammatory skin disease characterized by highly pruritic, eczematous skin lesions. Various factors including the immunological and non-immunological abnormalities contribute to the pathogenesis and development of AD. Patients with AD generally have a familial predisposition of increasing in serum IgE antibodies to various allergens and development of eczematous skin lesions as the immunological abnormalities. On the other hand, physiological barrier dysfunctions of the skin, high detection of Staphylococcus aureus (SA) in the bacterial flora on their skin surface flora and an increased hypersensitivity to itch are known as the non-immunological abnormalities. In this paper, we would like to review the recent papers concerning the pathogenesis and biomarker of AD and its itch and to discuss a probable role of SA, interleukin-18 (IL-18), nerve growth factor (NGF) and semaphorin 3A (Sema3A) of an axon guidance molecule in pathogenesis and treatment of AD.
Recently, we measured the conductance and transepidermal water loss (TEWL) on the medical examination of infant. In order to examine their usefulness for early diagnosis of AD and reveal that the TEWL in the uninvolved skin of abdomen significantly increased in infants with AD already as early as 6 months and 1.5 years old, when compared with that in infants without AD. Therefore, the measurement of TEWL in the skin is useful for early diagnosis of infantile AD and the early care on the skin of these infants might be expected to effect protectively on development and aggravation of AD.1 We have already reported that Staphylococcus aureus (SA) usually existed not only on the skin regions affected by AD but also on the atopic dry skin regions.2 The number of SA detected on the skin surface of forearm and forehead in AD patients was remarkably more than that in healthy controls, as shown Fig. 1.2 There was a significant correlation between eruption score and the number of SA detected on the same area of patients with AD (relationship coefficiency= 0.54, P<0.01),1 as shown in Fig. 2.3 We have also reported that farnesol (F) of a perfume and xylitol (X) of sugar alcohol synergistically can inhibit the biofilm formation of SA and moreover can dissolve the formed biofilm of SA, as shown in Fig. 3-1 and Fig. 3-2.3,4 Indeed, the treatment with FX cream improved significantly 4 out of 5 items in AD lesions at 2 weeks later, while the treatment with control cream improved only 1 item of dryness/desquamation as shown in Fig. 4.3,4 Both of the number of SA and the ratio of SA to total bacteria at the sites, to which the FX cream containing F and X had been applied, significantly decreased in 2 weeks later, accompanied with clinical improvement of AD, as shown in Fig. 5.3,4 The therapeutic effect of this FX cream is conceivable to be based on two functions of it, namely the prevention of adhesion of SA to the skin and the removal of SA from the skin. Therefore, this FX cream is expected as a useful skin care agent for atopic dry skin colonized by SA.3,4 Recently, we also found that AD-like lesions in DS-Nh of AD model mouse mice were significantly improved together with a decrease in number of SA detected from these lesions by treatment with the bamboo leaf extract cream having the antibacterial, viral and mycotic activity.5 This improvement effect was also observed together with a decrease of the increased serum IgE levels and IFN-γ/IL-13 production in Con A-stimulated cultures of lymph node cells taken from these mice. From these results, this extract cream is suggested to have not only antibacterial effects but also immunological effects as the therapeutic agent.5
It is well-known that SA discharges various toxins and enzymes that injure the skin, results in activation and proliferation of epidermal keratinocytes, which produce and release IL-18. The IL-18 is supposed to be involved in pathogenesis of AD, because IL-18 induces the super Th1 cells producing and secreting IFN-γ and IL-13.6 Indeed, the serum IL-18 levels in patients with AD significantly correlated with skin scores of AD lesions, as shown Fig. 6-1.7 From these lesions, SA is detected with high frequency and a correlationship is observed between eruption score and the number of SA detected, as above-mentioned. Also, there is a strong positive correlation between skin scores and serum IL-18 levels in DS-Nh mice (P<0.0001, r=0.64) with AD-like lesions, in which SA is supposed to be a critical factor for the development of AD lesions, as shown Fig. 6-2.7,8
DS-Nh mice develop AD-like legions when they are housed under conventional conditions, but develop skin legions with less severity and less frequency under specific pathogens free (SPF) conditions.9 Furthermore, an increase of IL-18 production from epidermal cells was observed in AD model mice induced by subsequent topical application of SA products.6 Therefore, in order to clarify the role of IL-18 in the pathogenesis of AD, we measured IL-18 levels in the horny layer (i.e. horn IL-18) by the method using tape stripping and ELISA, which has been already established for measurement of NGF level in the horny layer10 and assessed the horn IL-18 level in relation to clinical severities and SA colonization on the involved skin of AD patients. The horn proteins were collected via tape stripping from the horny layer of the skin in 61 AD patients and 40 healthy controls, and the horn IL-18 levels were measured using ELISA. Clinical severity, blood data and SA colonization of involved skin were also evaluated before and 4-8 weeks after treatment. The results showed that the horn IL-18 levels of skin lesions were significantly higher in AD patients than in healthy controls and correlated to SCORAD, serum IL-18, IgE, LDH, thymus and activation-regulated chemokine (TARC) and TEWL.11 In the group of AD patients with IgE <1,500 IU/m, the horn IL-18 levels was significantly higher in patients whom SA was detected than in patients from whom SA was not.11 From these results, SA colonization on the skin lesions is conceivable to contribute to the IL-18 production of epidermal keratinocytes especially in the group of AD patients with relatively low IgE production and the horn IL-18 levels seem to be associated with the severity of AD.
It is interesting that DS-Nh mice pretreated with cyclophosphamide (Cy) developed more remarkable AD-like lesions with more number of SA colonies on the skin surface, in comparison with untreated ones.12 The levels of IFN-γ and IL-13 in the supernatants of the lymph node cell cultures stimulated with SEB or ConA were increased remarkably in the Cy-treated mice, although the serum levels of total IgE were not.12 In this experiment, we also revealed that Cy-treated mice, to which CD25+ CD4+ regulatory T cells taken from non-treated ones had been transferred, developed AD-like legions with less severity and less number of SA colonies on the skin surface.12 Table simply shows the summary of these results. Therefore, it is presumed that CD25+CD4+ regulatory T cells might be involved in suppression of super Th1 cells, which are induced by IL-18 and are involved in the development of AD-like lesions rather than IgE production. The efficient induction of CD25+CD4+ regulatory T cells may become new type of treatment for recalcitrant AD. Fig. 7 shows schematically the action mechanism of FoxP3+CD25+CD4+ regulatory T cells, which are conceivable to suppress the induction and exacerbation of AD-like lesions through the suppression of IFN-γ/L-13 production of by FoxP3-CD25-CD4+ super Th1 cells in DS-Nh of AD model mouse associated with SA colonization on the skin surface.12-14
Nerve growth factor (NGF), which belongs to the neurotrophin family, is mainly produced and released by the basal keratinocytes15-19 and has diverse activity in the skin. Immunohistochemistry of human skin shows that NGF is expressed in suprabasal keratinocytes and in basal keratinocytes.18 Recently, neurogenic inflammation, including effect of neuropeptides such as substance P (SP) and NGF, has provided a new perspective in understanding the pathogenesis of AD.20 Increasing evidence suggests that neurotrophins are supposed to be involved in the neurogenic inflammation of AD and to be one of factors, which regulate development of AD.21 It has been reported that NGF levels of AD patients are measured in plasma,16 urine and saliva,22 and the NGF level in plasma increases and correlates with the disease severity.16 Furthermore, it has been also reported that expression of NGF increases in the AD lesions biopsied and relates to the aggravation of disease.23 However, it is not so good method to measure the NGF level in the biopsied skin samples because it is invasive and cannot be done repeatedly. By the preliminary experiments, we established a method using tape stripping and ELISA for measurement of NGF in the horny layer (i.e. horn NGF), which is able to be accepted repeatedly in the same patient and then measured the horn NGF in AD patients by this method.10 The results showed that the horn NGF level in the non-lesional skin of AD patients as well as in the lesional skin significantly increased, in comparison with the non-lesional skin of healthy subjects, as shown Fig. 8, being consistent with the previous histochemical findings that NGF expression in keratinocytes is increased in AD patients.10 Then, we assessed a possible relationship between the horn NGF level and the severity of itch and AD, and also examined effect of treatment on the horn NGF level. The positive correlations were observed between horn NGF level and itch severity, eruption score such as erythema, scale/xerosis and total skin score on the skin lesions measured, eosinophil count and serum LDH level, but were not between horn NGF level and skin scores of papule, erosion/crust and lichenification on the skin lesions measured and serum IgE level.10 The horn NGF level decreased significantly after treatment, which correlated with the decrease in itch severity, skin scores (erythema, papule, scale/xerosis, lichenification and total skin score) on the measured skin lesions, serum LDH level, and especially eosinophil count.10 These findings indicate that the horn NGF level reflects to some extent the severity of itch, eruptions, and laboratory data. The level of horn NGF had decreased significantly not only at 4 weeks, but also at 2 weeks after beginning treatment.10 Considering the fact that the turn-over of the horny layer is approximately 14 days, it is presumed that epidermal NGF production is suppressed immediately after beginning treatment. Also, a significant decrease in NGF level was observed not only in the group treated with olopatadine and topical steroid, but also in the group treated with olopatadine alone, as shown Fig. 9. How olopatadine suppresses the increase of horn NGF levels induced in AD patients? It has been reported that histamine enhances NGF production of human keratinocyte through the stimulation of H1 receptors.24 Therefore, H1-antagtonist including olopatadine is conceivable to suppress the increase of horn NGF level by inhibiting the enhancement of NGF production by keratinocyte stimulated with histamine. Also, we have already reported that olopatadine suppresses the increase of SP levels in the skin lesions induced by the repeated application of 2,4,6-trinitrochlorbenzane in DSNh of atopic model mouse.25 In AD patients, too, it has been reported that olopatadine significantly decreased the serum SP level because of down-modulatory effect on tachykinin release.26 Other studies have also shown that whereas NGF stimulates the synthesis and releases of SP in the central and peripheral nervous system, SP induces NGF synthesis via cytokines, too.27,28 These results suggest that the activations of NGF and SP are interdependent. Moreover, in studies of BALB/c mice with chronic contact hypersensitivity induced by oxazolone, it has been reported that oral administration of olopatadine suppressed the production of NGF and cytokines such as IL-4, IL-1β, IL-6 and GM-CSF in the affected ear tissues, unlike other histamine H1 receptor antagonists.29-31 From these results, olopatadine is suggested to exerts its effect on AD not only by antihistamine effect but also by suppressing the production of NGF and SP. Taken all together, the horn NGF level is presumed to be able to serve as a marker to evaluate the clinical conditions of AD and the immediate effect of treatment because it can reflect the severity of itch and eruptions in AD. Therefore, quantification of NGF in the samples collected directly from the horny layer appears to be useful in assessing the severity of AD and the therapeutic effects on AD.
Topical steroids and antihistamines are generally used for the treatment of AD. However, these treatments are not effective sufficiently for the recalcitrant AD with severe itching followed by uncontrollable scratching. In AD patients, the C-fiber in the epidermis increases and sprouts with the increase of horn NGF levels, inducing hypersensitivity to itching, which is considered to aggravate the disease, as above-mentioned. Semaphorin 3A (Sema3A), an axon guidance molecule, is a potent inhibitor of neurite outgrowth of sensory neurons by exerting the plexin-A1-4 and neuropilin-1 (NRP-1) of its receptors.32,33 Fig. 10 shows semaphorin family and its receptors. Sema3A may become effective drug against refractory itching skin diseases such as AD in future by suppressing the hypersensitivity to itching through inhibition of the extension and sprout of C-fiber in the epidermis. From this point of view, we administered the recombinant Sema3A intracutaneously into the AD-like skin lesions of NC/Nga mice, an animal model of AD, in order to investigate the effect of Sema3A on AD. Sema3A improved significantly AD-like lesions of NC/Nga, AD model mouse (Fig. 11-1), and the improvement effect was a dose-dependent manner (Fig. 11-2) and was presumed to be brought through the suppression of scratching accompanied with itching, because Sema3A suppressed significantly the scratching behavior of NC/Nga mice (Fig. 11-3). Histopathological examinations revealed an improvement of acanthosis, a remarkable reduction in the density of invasive nerve fibers, and a decrease of the inflammatory infiltrate including mast cells and CD4+ T cells and the interleukin-4 (IL-4) production in the Sema3A-injected area of AD-like lesions.34 The improvement effect was associated with the suppression of scratching behavior in Sema3A-treated animals. These findings indicate for the first time that Sema3A in vivo remarkably improves AD-like lesions in NC/Nga mice, an animal model of AD. This is the first trial for a potential clinical application of semaphorins. Sema3A acts locally and exerts a continuous effect for a while even after discontinuation. But this effect of Sema3A on scratching was reversible, because the decrease in scratching behavior returned to the basal levels immediately after the discontinuation of Sema3A, followed by recurrence of lesions, as shown in Fig. 11-1, 2, 3. These results suggest that scratching behavior aggravates AD and Sema3A can ameliorate the symptoms of AD by interrupting the itch-scratch cycle, which is an important cause of aggravation and chronicity of AD. Recently, there has been an interesting report that epidermal Sema3A levels were decreased in patients with AD compared with healthy volunteers, concomitant with the increase of epidermal nerve densities.35 This result shows that there is a good correlation between epidermal innervation and Sema3A levels, which may provide an important evidence for the therapeutic claim against itching. Many drugs have been developed for the treatment of AD. However there is no treatment other than Sema3A which targets the invasive nerve fibers in the epidermis. Therefore, Sema3A is expected to become an effective and useful drug for AD patients with severe recalcitrant itching, which is resistant to anti-histamine drugs of H1-blocker and anti-inflammatory steroid ointment.
Recently, transient receptor potential vanilloid receptor subtype 1 (TRPV1) and capsaicin are noticed as topics regarding treatment of itching. The generally accepted concept for the therapeutic application of capsaicin to mitigate itch is based on desensitizing effect of this vanilloid. However, the most notorious clinical limitation of capsaicin application is supposed to be acute excitation of the sensory C-afferents, which is induced by the TRPV1 and results in a remarkable "hot painful burning" sensation.36 Does reduction in innervation density of intraepidermal nerve fibers by the local application of Sema3A cause such a sensation? In our experiments regarding the effects of Sema3A on AD-like lesions, no apparent abnormalities in antinociceptive response as well as in general symptoms were observed on local treatment with Sema3A. Answering above questions should be required before therapeutic attempts in human. Sema3A, when intracutaneously administered repeatedly, significantly improved AD lesions on day 2 or 3 after the start of the injections, as shown in Fig. 11-3. Recent studies indicate that semaphorins play diverse roles unrelated to axon guidance, including organogenesis, vascularization and angiogenesis.37-39 In particular, attention has been given to the fact that several semaphorins play critical roles in the immune system.40,41 It has been reported that Sema3A,41,42 Sema3C,43 Sema4A,44 Sema4D42,44-50 and Sema7A51 function in various phases of immune system. From these recent knowledges and the above-mentioned histopathological findings in our experiments, the effects of Sema3A on AD-like lesions are conceivable to involve other mechanisms such as regulation of the immune and vascular systems, in addition to the suppression of the invasive sensory neurons in the epidermis. In addition, Sema3A and NRP-1 of its receptors expressed in human keratinocytes52 may be relevant to the above-mentioned suppressive effect of Sema3A on acanthosis observed in our present experiment. Also Sema3A and NRP-1 of its receptors expressed in dendritic cells and T cells53,54 may be relevant to the immunological effect of Sema3A such as suppression of CD4+ T cells infiltration and IL-4 production observed in the AD-like lesions in NC/Nga mice treated with Sema3A, as above-mentioned.
In 2005, Takano et al., reported that anti-NGF antibodies inhibited the development of skin lesions and epidermal innervation in the NC/Nga of AD model mouse, accompanied with the reduction of scratching.55,56 Thus Sema3A may function as well as anti-NGF antibodies may act on the both of immunological and neurological mechanisms. However, the combination therapy of Sema3A with anti-NGF antibody may not be justified for AD, because NGF is known to increase the expression levels of NRP-157 and to augment the effect of Sema3A to induce axon repulsion (or growth cone collapse) of dorsal root ganglia neurons.58 Further studies should be required to elucidate the mechanism of action of Sema3A on AD. Fig. 12 shows schematically the pathomechanism of itch and action mechanism of Sema3A on itching of AD lesions. Sema3A has not only the neurological activity, which inhibits the intraepidermal extension of peripheral nerve, but also the immunological anti-inflammatory activity, and is expected as a new type of drug effective for refractory AD patients who are resistant to existing drugs. It is possible that Sema3A is also widely effective for severe itching skin diseases other than AD in which the itchscratch cycle is involved.
Figures and Tables
 | Fig. 1The number of SA detected on the skin surface of forearm and forehead in patients with AD is remarkably more than in healthy controls.2 cfu: colony forming unit. |
 | Fig. 2The correlation between eruption score and the number of SA on the same area of patients with AD.3 cfu: colony forming unit. |
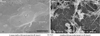 | Fig. 3-1Farnesol and xylitol (FX) have an inhibitory effect on biofilm formation of SA.3 A biofilm of bacteria was formed by incubating a plastic coverslip coated with type IV collagen in each well of a 24-well tissue culture plate with the human plasma and TSB (1:1) medium, into which SA was inoculated. Cell suspensions of SA from an AD patient (1×108 cfu/mL) were inoculated separately into 1 mL of the medium either alone (control) or supplemented with FX. After incubation at 37℃, each coverslip was observed visually and also under a scanning electron microscope (SEM) and number of SA colony adhered to the coverslip of was counted. TSB: tryptic soy broth. |
 | Fig. 3-2FX dissolve the formed biofilm of SA.3
|
 | Fig. 4The treatment with FX cream improved significantly 4 items out of 5 items in AD lesions 2 weeks later, while the treatment with control cream improved only 1 item of dryness/desquamation.3
|
 | Fig. 5Application of FX cream containing 0.2% farnesol and 5% xylitol to AD patients for 2 weeks decreased the number and rate of SA significantly when compared with the number at the start and with placebo control treatment.3 FX: cream containing 0.2% farnesol and 5% xylitol, CT: control treatment, #the rate (%) of SA to total bacteria, N.S. : not significant, **P < 0.05, ***P < 0.01. |
 | Fig. 6-1Comparison between serum levels of IL-18 and global skin scores in AD patients divided into 4 groups based on their disease severities.7
|
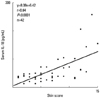 | Fig. 6-2Comparison between serum IL-18 levels and skin scores in DS-Nh mice (n=14 each group).7
|
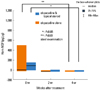 | Fig. 9Effect of treatment on horny NGF of AD patients.10
|
 | Fig. 10Semaphorin family and its receptorsSema.33,34 D: Semadomain, Ig: immunoglobulin, BD: Basic domain, TSP: Thrombospondin repeat, GPI: Glycerophosphatidyl-inositol anchor, MRS: Met-related sequence, G-P: glycine proline repeat, CUB: complement binding domains (a1 and a2 domains), FV/VIII: FactorV/VIII coagulation factor-like domains (b1 and b2 domains), MAM: Mepulin-A5-µ domain. |
 | Fig. 11-1Sema3A improves AD-like lesions of NC/Nga, AD model mouse.34 The clinical skin score was defined as the sum of individual scores graded as 0 (none), 1 (mild), 2 (moderate) and 3 (severe) for the symptoms of erythema/hemorrhage, edema, excoriation/erosion and scaling/dryness. *P < 0.05, **P < 0.01, compared to no treatment group and control group. |
 | Fig. 11-2Sema3A improves clinical skin score in a dose-dependent manner.34 The clinical skin score was defined as the sum of individual scores graded as 0 (none), 1 (mild), 2 (moderate) and 3 (severe) for the symptoms of erythema/hemorrhage, edema, excoriation/erosion and scaling/dryness. *P < 0.05, **P < 0.01, compared to no treatment group and control group. |
 | Fig. 11-3Sema3A suppresses scratching behavior of NC/Nga mice.34
|
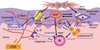 | Fig. 12Pathomechanism of itch and action mechanism of Sema3A on itching of AD lesions: Sema3A has not only the neurological activity, which inhibits the intraepidermal extension of peripheral nerve, but also the immunological anti-inflammatory activity, and is expected as a new type of drug effective for refractory AD patients who are resistant to existing drugs. |
ACKNOWLEDGMENTS
This work was partly supported by a Grant-in-Aid for 2004-2011 Scientific Research from the Ministry of Education, Culture, Sports and Technology (project No.16591108, No.16591320, No.21591471: Z. Ikezawa) and 2003-2008 Scientific Research from the Ministry of Health, Labour and Welfare of Japan.
References
1. Kanbara T, Nakamura K, Tanaka Y, Inoue Y, Tanaka K, Ikezawa Z. A study about the prevalence of infantile atopic dermatitis (the 5th report). Proceedings in Arerugi. 2009. 58:376.
2. Katsuyama M, Wachi Y, Kitamura K, Suga C, Onuma S, Ikezawa Z. Correlation between the population of staphylococcus aureus on the skin and severity of score of dry type atopic dermatitis condition. Nippon Hifuka Gakkai Zasshi. 1997. 107:1103–1111.
3. Katsuyama M, Ichikawa H, Ogawa S, Ikezawa Z. A novel method to control the balance of skin microflora. Part 1. Attack on biofilm of Staphylococcus aureus without antibiotics. J Dermatol Sci. 2005. 38:197–205.
4. Katsuyama M, Kobayashi Y, Ichikawa H, Mizuno A, Miyachi Y, Matsunaga K, Kawashima M. A novel method to control the balance of skin microflora Part 2. A study to assess the effect of a cream containing farnesol and xylitol on atopic dry skin. J Dermatol Sci. 2005. 38:207–213.
5. Fujita H, Takahashi H, Aihara M, Hirasawa T, Ikezawa Z. Effects of bamboo leaf extract on atopic dermatitis model mice. J Environ Dermatol. 2006. 13:87–94.
6. Terada M, Tsutsui H, Imai Y, Yasuda K, Mizutani H, Yamanishi K, Kubo M, Matsui K, Sano H, Nakanishi K. Contribution of IL-18 to atopic-dermatitis-like skin inflammation induced by Staphylococcus aureus product in mice. Proc Natl Acad Sci U S A. 2006. 103:8816–8821.
7. Kirino M, Kirino Y, Takeno M, Nagashima Y, Takahashi K, Kobayashi M, Murakami S, Hirasawa T, Ueda A, Aihara M, Ikezawa Z, Ishigatsubo Y. Heme oxygenase 1 attenuates the development of atopic dermatitis-like lesions in mice: implications for human disease. J Allergy Clin Immunol. 2008. 122:290–297. 7 e1-8.
8. Matsukura S, Aihara M, Hirasawa T, Ikezawa Z. Effects of TNCB sensitization in DS-Nh mice, serving as a model of atopic dermatitis, in comparison with NC/Nga mice. Int Arch Allergy Immunol. 2005. 136:173–180.
9. Yoshioka T, Hikita I, Matsutani T, Yoshida R, Asakawa M, Toyosaki-Maeda T, Hirasawa T, Suzuki R, Arimura A, Horikawa T. DS-Nh as an experimental model of atopic dermatitis induced by Staphylococcus aureus producing staphylococcal enterotoxin C. Immunology. 2003. 108:562–569.
10. Yamaguchi J, Aihara M, Kobayashi Y, Kambara T, Ikezawa Z. Quantitative analysis of nerve growth factor (NGF) in the atopic dermatitis and psoriasis horny layer and effect of treatment on NGF in atopic dermatitis. J Dermatol Sci. 2009. 53:48–54.
11. Inoue Y, Aihara M, Harada I, Komori J, Kirino M, Ikezawa Z. Analysis of the pathogenesis by the quantitative measurement of interleukin-18 in the horny layer and Staphylococcus aureus colonization on the skin surface in patients with atopic dermatitis. Proceedings in Arerugi. 2009. 58:1262.
12. Ikezawa Y. Exacerbative effect of cyclophosphamide on symptom of atopic dermatitis in DS-Nh mouse. Yokohama Medical Journal. 2004. 55:437–443.
13. Ikezawa Y, Nakazawa M, Tamura C, Takahashi K, Minami M, Ikezawa Z. Cyclophosphamide decreases the number, percentage and the function of CD25+ CD4+ regulatory T cells, which suppress induction of contact hypersensitivity. J Dermatol Sci. 2005. 39:105–112.
14. Tatewaki S, Nakazawa M, Aihara M, Hongo N, Hotta Ch, Ikezawa Y, Takahashi K, Minami M, Ikezawa Z. Effects of cyclophosphamide on 2, 4-dinitrofluorobenzene-specific oral tolerance. J Environ Dermatol Cutan Allergol. 2007. 1:12–21.
15. Yoshioka T, Hikita I, Asakawa M, Hirasawa T, Deguchi M, Matsutani T, Oku H, Horikawa T, Arimura A. Spontaneous scratching behaviour in DS-Nh mice as a possible model for pruritus in atopic dermatitis. Immunology. 2006. 118:293–301.
16. Toyoda M, Nakamura M, Makino T, Hino T, Kagoura M, Morohashi M. Nerve growth factor and substance P are useful plasma markers of disease activity in atopic dermatitis. Br J Dermatol. 2002. 147:71–79.
17. Pincelli C, Sevignani C, Manfredini R, Grande A, Fantini F, Bracci-Laudiero L, Aloe L, Ferrari S, Cossarizza A, Giannetti A. Expression and function of nerve growth factor and nerve growth factor receptor on cultured keratinocytes. J Invest Dermatol. 1994. 103:13–18.
18. Botchkarev VA, Yaar M, Peters EM, Raychaudhuri SP, Botchkareva NV, Marconi A, Raychaudhuri SK, Paus R, Pincelli C. Neurotrophins in skin biology and pathology. J Invest Dermatol. 2006. 126:1719–1727.
19. Pincelli C, Marconi A. Autocrine nerve growth factor in human keratinocytes. J Dermatol Sci. 2000. 22:71–79.
20. Huang CH, Kuo IC, Xu H, Lee YS, Chua KY. Mite allergen induces allergic dermatitis with concomitant neurogenic inflammation in mouse. J Invest Dermatol. 2003. 121:289–293.
21. Peters EM, Raap U, Welker P, Tanaka A, Matsuda H, Pavlovic-Masnicosa S, Hendrix S, Pincelli C. Neurotrophins act as neuroendocrine regulators of skin homeostasis in health and disease. Horm Metab Res. 2007. 39:110–124.
22. Masahiko T. Useful therapeutic markers of atopic dermatitis: neurogenic factors. Skin Research. 2005. 4:Suppl 5. 87–93.
23. Dou YC, Hagstromer L, Emtestam L, Johansson O. Increased nerve growth factor and its receptors in atopic dermatitis: an immunohistochemical study. Arch Dermatol Res. 2006. 298:31–37.
24. Kanda N, Watanabe S. Histamine enhances the production of nerve growth factor in human keratinocytes. J Invest Dermatol. 2003. 121:570–577.
25. Kojima M, Aihara M, Yamada M, Matsukura S, Hirasawa T, Ikezawa Z. Effects of neuropeptides in the development of the atopic dermatitis of mouse models. Allergol Int. 2004. 53:169–178.
26. Izu K, Tokura Y. The various effects of four H1-antagonists on serum substance P levels in patients with atopic dermatitis. J Dermatol. 2005. 32:776–781.
27. Lindsay RM, Harmar AJ. Nerve growth factor regulates expression of neuropeptide genes in adult sensory neurons. Nature. 1989. 337:362–364.
28. Braun A, Appel E, Baruch R, Herz U, Botchkarev V, Paus R, Brodie C, Renz H. Role of nerve growth factor in a mouse model of allergic airway inflammation and asthma. Eur J Immunol. 1998. 28:3240–3251.
29. Tamura T, Matsubara M, Takada C, Hasegawa K, Suzuki K, Ohmori K, Karasawa A. Effects of olopatadine hydrochloride, an antihistamine drug, on skin inflammation induced by repeated topical application of oxazolone in mice. Br J Dermatol. 2004. 151:1133–1142.
30. Tamura T, Matsubara M, Hasegawa K, Ohmori K, Karasawa A. Olopatadine hydrochloride suppresses the rebound phenomenon after discontinuation of treatment with a topical steroid in mice with chronic contact hypersensitivity. Clin Exp Allergy. 2005. 35:97–103.
31. Tamura T, Amano T, Ohmori K, Manabe H. The effects of olopatadine hydrochloride on the number of scratching induced by repeated application of oxazolone in mice. Eur J Pharmacol. 2005. 524:149–154.
32. Kruger RP, Aurandt J, Guan KL. Semaphorins command cells to move. Nat Rev Mol Cell Biol. 2005. 6:789–800.
33. Neufeld G, Kessler O. The semaphorins: versatile regulators of tumour progression and tumour angiogenesis. Nat Rev Cancer. 2008. 8:632–645.
34. Yamaguchi J, Nakamura F, Aihara M, Yamashita N, Usui H, Hida T, Takei K, Nagashima Y, Ikezawa Z, Goshima Y. Semaphorin3A alleviates skin lesions and scratching behavior in NC/Nga mice, an atopic dermatitis model. J Invest Dermatol. 2008. 128:2842–2849.
35. Tominaga M, Ogawa H, Takamori K. Decreased production of semaphorin 3A in the lesional skin of atopic dermatitis. Br J Dermatol. 2008. 158:842–844.
36. Biro T, Toth BI, Marincsak R, Dobrosi N, Geczy T, Paus R. TRP channels as novel players in the pathogenesis and therapy of itch. Biochim Biophys Acta. 2007. 1772:1004–1021.
37. Kitsukawa T, Shimono A, Kawakami A, Kondoh H, Fujisawa H. Overexpression of a membrane protein, neuropilin, in chimeric mice causes anomalies in the cardiovascular system, nervous system and limbs. Development. 1995. 121:4309–4318.
38. Behar O, Golden JA, Mashimo H, Schoen FJ, Fishman MC. Semaphorin III is needed for normal patterning and growth of nerves, bones and heart. Nature. 1996. 383:525–528.
39. Soker S, Takashima S, Miao HQ, Neufeld G, Klagsbrun M. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell. 1998. 92:735–745.
40. Kikutani H, Kumanogoh A. Semaphorins in interactions between T cells and antigen-presenting cells. Nat Rev Immunol. 2003. 3:159–167.
41. Lepelletier Y, Smaniotto S, Hadj-Slimane R, Villa-Verde DM, Nogueira AC, Dardenne M, Hermine O, Savino W. Control of human thymocyte migration by Neuropilin-1/Semaphorin-3A-mediated interactions. Proc Natl Acad Sci U S A. 2007. 104:5545–5550.
42. Delaire S, Billard C, Tordjman R, Chedotal A, Elhabazi A, Bensussan A, Boumsell L. Biological activity of soluble CD100. II. Soluble CD100, similarly to H-SemaIII, inhibits immune cell migration. J Immunol. 2001. 166:4348–4354.
43. Mangasser-Stephan K, Dooley S, Welter C, Mutschler W, Hanselmann RG. Identification of human semaphorin E gene expression in rheumatoid synovial cells by mRNA differential display. Biochem Biophys Res Commun. 1997. 234:153–156.
44. Kumanogoh A, Marukawa S, Suzuki K, Takegahara N, Watanabe C, Ch'ng E, Ishida I, Fujimura H, Sakoda S, Yoshida K, Kikutani H. Class IV semaphorin Sema4A enhances T-cell activation and interacts with Tim-2. Nature. 2002. 419:629–633.
45. Bougeret C, Mansur IG, Dastot H, Schmid M, Mahouy G, Bensussan A, Boumsell L. Increased surface expression of a newly identified 150-kDa dimer early after human T lymphocyte activation. J Immunol. 1992. 148:318–323.
46. Herold C, Bismuth G, Bensussan A, Boumsell L. Activation signals are delivered through two distinct epitopes of CD100, a unique 150 kDa human lymphocyte surface structure previously defined by BB18 mAb. Int Immunol. 1995. 7:1–8.
47. Hall KT, Boumsell L, Schultze JL, Boussiotis VA, Dorfman DM, Cardoso AA, Bensussan A, Nadler LM, Freeman GJ. Human CD100, a novel leukocyte semaphorin that promotes B-cell aggregation and differentiation. Proc Natl Acad Sci U S A. 1996. 93:11780–11785.
48. Wu Y, Nadler MJ, Brennan LA, Gish GD, Timms JF, Fusaki N, Jongstra-Bilen J, Tada N, Pawson T, Wither J, Neel BG, Hozumi N. The B-cell transmembrane protein CD72 binds to and is an in vivo substrate of the protein tyrosine phosphatase SHP-1. Curr Biol. 1998. 8:1009–1017.
49. Kumanogoh A, Watanabe C, Lee I, Wang X, Shi W, Araki H, Hirata H, Iwahori K, Uchida J, Yasui T, Matsumoto M, Yoshida K, Yakura H, Pan C, Parnes JR, Kikutani H. Identification of CD72 as a lymphocyte receptor for the class IV semaphorin CD100: a novel mechanism for regulating B cell signaling. Immunity. 2000. 13:621–631.
50. Watanabe C, Kumanogoh A, Shi W, Suzuki K, Yamada S, Okabe M, Yoshida K, Kikutani H. Enhanced immune responses in transgenic mice expressing a truncated form of the lymphocyte semaphorin CD100. J Immunol. 2001. 167:4321–4328.
51. Holmes S, Downs AM, Fosberry A, Hayes PD, Michalovich D, Murdoch P, Moores K, Fox J, Deen K, Pettman G, Wattam T, Lewis C. Sema7A is a potent monocyte stimulator. Scand J Immunol. 2002. 56:270–275.
52. Kurschat P, Bielenberg D, Rossignol-Tallandier M, Stahl A, Klagsbrun M. Neuron restrictive silencer factor NRSF/REST is a transcriptional repressor of neuropilin-1 and diminishes the ability of semaphorin 3A to inhibit keratinocyte migration. J Biol Chem. 2006. 281:2721–2729.
53. Tordjman R, Lepelletier Y, Lemarchandel V, Cambot M, Gaulard P, Hermine O, Romeo PH. A neuronal receptor, neuropilin-1, is essential for the initiation of the primary immune response. Nat Immunol. 2002. 3:477–482.
54. Lepelletier Y, Moura IC, Hadj-Slimane R, Renand A, Fiorentino S, Baude C, Shirvan A, Barzilai A, Hermine O. Immunosuppressive role of semaphorin-3A on T cell proliferation is mediated by inhibition of actin cytoskeleton reorganization. Eur J Immunol. 2006. 36:1782–1793.
55. Takano N, Arai I, Kurachi M. Analysis of the spontaneous scratching behavior by NC/Nga mice: a possible approach to evaluate antipruritics for subjects with atopic dermatitis. Eur J Pharmacol. 2003. 471:223–228.
56. Takano N, Sakurai T, Kurachi M. Effects of anti-nerve growth factor antibody on symptoms in the NC/Nga mouse, an atopic dermatitis model. J Pharmacol Sci. 2005. 99:277–286.
57. Pond A, Roche FK, Letourneau PC. Temporal regulation of neuropilin-1 expression and sensitivity to semaphorin 3A in NGF- and NT3-responsive chick sensory neurons. J Neurobiol. 2002. 51:43–53.
58. Messersmith EK, Leonardo ED, Shatz CJ, Tessier-Lavigne M, Goodman CS, Kolodkin AL. Semaphorin III can function as a selective chemorepellent to pattern sensory projections in the spinal cord. Neuron. 1995. 14:949–959.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download