Abstract
New information regarding the molecular mechanisms of allergic disorders has led to a variety of novel therapeutic approaches. This article briefly reviews the pathogenesis of asthma and allergic diseases, discusses the rationale behind using immunomodulators in these diseases; and examines the therapeutic effects of immunomodulators on allergic diseases. There are a number of immunomodulators that have been developed for the treatment of allergic disorders. Some have looked very promising in pre-clinical trials, but have not shown significant benefits in human clinical trials thus indicating the disparity between mouse models and human asthma. This review focuses on immunomodulators that are in human clinical trials and not molecules in pre-clinical development.
New information regarding the molecular mechanisms of allergic disorders has led to a variety of novel therapeutic approaches. This article will briefly review the pathogenesis of asthma and allergic diseases; discuss the rationale behind using immunomodulators in these diseases; and examine the therapeutic effects of immunomodulators on allergic diseases. This review will focus on immunomodulators that are in human clinical trials and not molecules in pre-clinical development.
One might question why new treatments are needed when with the advent of new and better inhaled corticosteroids with and without long-acting β-agonists many individuals with asthma are well controlled. However, as with all treatments, inhaled corticosteroids are not effective for all individuals. Indeed, about 30%-35% of individuals, both adult and pediatric patients with asthma, have a poor or no response to inhaled corticosteroids. Furthermore, inhaled corticosteroids have not been shown to prevent the progression of disease or completely reverse airway remodeling.1,2
Thus, there is a need for novel therapies that affect critical immunopathologic mechanisms and that would induce immune tolerance; that is, change the immune system such that the therapy might actually be able to be discontinued with continued maintenance of disease control long term.
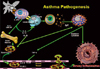
The pathogenesis of asthma involves a number of different cells, mediators, and cytokines (Figure). It is also apparent that particular pathways or molecules may be more important in individual patients. Thus, finding a universal treatment that would be clinically beneficial for all patients is a challenge.
One of the first steps important in the pathogenesis of allergic respiratory diseases is antigen capture and presentation. This is followed by engagement of the T-cell receptor and CD3 complex by MHC class II molecules on B cells. Subsequently, there is a chain of molecular events including engagement of CD154 (CD40L) on Th2 cells and CD40 on B cells, along with engagement of CD28 and CD80/86 on Th2 and B cells respectively. These events ultimately lead to the release of important cytokines such as IL-4 and IL-13 critical for immunoglobulin E (IgE) production. Once IgE is produced, it binds to mast cells and basophils where cross-linking of high affinity IgE receptors bound by IgE and antigen leads to mediator and cytokine release. Important in this process is the release of more IL-4 and IL-13 from a number of different inflammatory cells including mast cells, as well as IL-9. These cytokines are important to propagate the production of IgE and allergic inflammation in general. IL-5 and GM CSF are also produced and these are key molecules for growth and differentiation of eosinophils. Eosinophils are key immune effector cells that can leave the blood vessel through the interactions of adhesion molecules and chemoattractants and migrate to the site of inflammation. This process involves a number of different molecules in addition to those mentioned above, many of which have been targets of therapeutic interventions.
Strategies to inhibit inflammatory pathways are many and include targeting the cell of origin of inflammatory cytokines and mediators, targeting the released mediator or cytokine, or inhibiting the effects of the released cytokine or mediator by blocking the mediated effects on the target cell. All of these approaches have been undertaken with various molecules. For example, soluble receptors and monoclonal antibodies against key cytokines such as IL-4 have undergone clinical trials. Inhibitors of target cell receptors for these molecules have also been studied.
This review will focus on many different approaches by examining the clinical effectiveness of novel immunomodulators involved in key pathogenic mechanisms.
Due to the pro-inflammatory role of activated T-cells in asthmatic airways and the observed correlation of increased CD25 expression with asthma severity, attempts have been made to block this arm of allergic disease pathogenesis. Initial attempts included monoclonal antibodies against Th2 cells such as keliximab. Although this treatment showed some modest improvement in patients with severe asthma, because of the potential side effects, it has not been extensively studied.
Cyclosporine and tacrolimus have similar mechanisms inhibiting T cells and have been used for the treatment of severe asthma. Cyclosporine in early studies was shown to improve pulmonary functions in severe asthmatics, but because of side effects it has not been used extensively. An inhaled form of tacrolimus was used in patients with asthma, but was unsuccessful in meeting primary endpoints.
Daclizumab is a humanized monoclonal IgG1 antibody that is currently approved for the prevention of renal allograft rejection. It is specific for CD25 expressed by activated T-cells and inhibits IL-2 stimulated proliferation by blocking the IL-2 receptor alpha chain. It does not deplete circulating CD25+ cells however. In a study by Busse et al. in 2008,3 moderate to severe asthma patients were placed on intravenous daclizumab. They showed improved pulmonary functions, reduced asthma symptoms and rescue medication use, increased time to severe exacerbations, and reduced peripheral blood eosinophil and serum eosinophil cationic protein levels. These investigators found greater activity in a refractory asthma subgroup. In that group, daclizumab resulted in approximately 10% improvement in FEV1 values, whereas patients on placebo had approximately 10% decrease in FEV1. There was also a two-thirds reduction in the number of asthma exacerbations in patients treated with daclizumab versus placebo. Thus far, a subcutaneous formulation has not been tried for asthma so it unclear whether or not this will be a potentially viable treatment for patients with asthma.
Another approach to target T lymphocytes and other key cells important in the pathogenesis of allergic respiratory diseases is to use ligands of toll-like receptors (TLR). Thirteen different toll-like receptors have been described in humans. Mycobacteria activate toll-like receptors 1 and 2, and the use of attenuated strains has been tried in humans, with minimal to no effect. There is much more information, however, on the use of TLR-4 and TLR-9 agonists.
The ligand for TLR-4 receptors is endotoxin/lipid A. A specific form of this, monophosphoryl lipid A (MPL) has been used as monotherapy as well as in combination with allergen immunotherapy for the treatment of allergic diseases. Ultra short course vaccines containing MPL have been used since the 1970's for seasonal allergic rhinitis from grass, tree or ragweed. The current preparation includes a glutaraldehyde-modified antigen absorbed onto L-tyrosine depot to enhance tolerability plus MPL to improve efficacy via activation of toll-4 receptors. Several trials have shown that four pre-seasonal injections of this preparation can reduce symptoms and medication use, elevate antigen-specific IgG and blunt seasonal elevations of IgE. Recent United States trials have shown positive results for both grass and ragweed allergies. In a study presented at the European Academy of Allergy and Clinical Immunology meeting in 2008, Zielen et al.,4 showed that an ultra short course specific immunotherapy with Polinex Quattro™ in pediatric and adolescent patients resulted in improvement in patients treated three consecutive years pre-pollen season. In addition, the amount of improvement in patients was greater in years 2 and 3. These data suggest that this approach of four pre-seasonal injections might be appropriate to use in controlling patients who have significant seasonal allergic rhinitis.
Immunostimulatory DNA molecules, CpG, have been used as well to treat allergic respiratory diseases. TLR-9 agonists have been used as monotherapy and conjugated to antigen in human clinical trials. Initial human experiments showed that immunostimulatory CpG molecules linked to the predominant short ragweed allergen, Amb a1, stimulates an antigen-specific Th2 to Th1 shift and improves the safety margin of allergen immunotherapy. In a study by Creticos et al.,5 twenty-five subjects with seasonal allergic rhinitis due to ragweed were given six preseasonal injections of Tolamba™, the combination of Amb a1 plus CpG conjugated to each other, in increasing doses from 0.06 to 12 mcg. The investigators found improvements in symptoms in the year the patients were treated as well as the subsequent year with no further treatment. There was an approximate 50% reduction in symptoms in both years with decreased rescue medication and improvement in quality of life. However, large scale clinical trials have been disappointing. Although there were technical difficulties with the larger studies that may account for the disappointing results, the utility of this approach has been questioned and further clinical trials are not currently planned.
However, another approach using a TLR-9 agonist has been shown to be effective. CYT003-QbG10, is a novel immunotherapy agent that consists of a virus-like particle, Qb and a short stretch of DNA from mycrobacteria that activates toll-like receptor 9 present in dendritic and other cells. In a recent publication by Senti et al.,6 CYT003-QbG10 plus house dust mite allergen was given for ten weeks to human subjects with asthma and allergic rhinitis. These investigators found improvements in symptom scores and quality of life for up to 48 weeks. Inhibition of skin test responses to house dust mite was also sustained for up to 48 weeks. This implies that long after discontinuing treatment there is sustained clinical improvement. Interestingly, in an eighty patient seasonal allergic rhinitis study, total rhinoconjunctivitis symptom scores were shown to be improved with QbG10 molecule without allergen.7 The treatment appeared safe and well tolerated, and efficacious in lowering rhinoconjunctivitis symptoms. Since this latter study did not use an allergen, it is concluded that CYT 003 acts through an allergen-independent mechanism and not through an adjuvant effect. A phase IIIb study with 300 patients suffering from perennial allergies has recently been started.
Another approach targeting T-cells and the early phases of allergic inflammation has been to use peroxisome proliferator-activated receptor (PPAR) agonists. This approach has been shown to be effective in murine models.8 PPAR-gamma agonists inhibit gene expression by binding to AP1 and other coactivators inhibiting the production of important inflammatory cytokines. PPAR-gamma agonists have a number of potential mechanisms which could be important.8 These include inhibition of cytokine production from a number of inflammatory cells in addition to T-lymphocytes and decreased chemotaxis of inflammatory cells. Human clinical trials are under way and it will be interesting to see whether these agents, already used for Type II diabetes mellitus (glitazones), might also be effective for asthma.
Because of the importance of Th-2 cytokines, IL-4, IL-5, IL-9, and IL-13, strategies aimed at inhibiting their production or effects have been tried in human clinical trials (Table). Initial attempts to use strategies aimed at IL-4 showed some promise, but subsequent large scale studies were unrewarding. This has included soluble IL-4 receptors and monoclonal antibodies against IL-4.
There are several strategies aimed at IL-4Rα, the common receptor chain important for both IL-4 and IL-13 signaling. AMG 317, is a humanized monoclonal antibody to IL-4Rα that has undergone initial human clinical trials.9 Early trials have been disappointing with some minor biologic activity but no clinical effects. Nonetheless, further development of this molecule is warranted since different routes and dosages may be important in showing clinical effectiveness. An inhaled anti-sense molecule to IL-4Rα, AIR645 has also undergone very early clinical trials, but no positive clinical effects have been reported thus far.
A 14 kD IL-4 mutein that blocks IL-4Rα has been shown to be effective for both atopic dermatitis when given subcutaneously and asthma when given by inhalation. The inhaled form of this molecule, pitrakinra (AEROVANT™) was reported to block early and late phase allergen responses in patients with asthma.10 Its efficacy in large scale clinical trials with traditional primary endpoints has yet to be determined.
Murine model studies have shown that IL-13 plays an important role in airway hyperresponsiveness, mucus production, secretion of eotaxin, and airway remodeling. There are several humanized monoclonal antibodies against IL-13 that are currently under development in phase I or phase II human clinical trials.
CAT-354 is a human monoclonal anti-IL-13 antibody that is in a phase IIa parallel-arm study to evaluate the efficacy and safety of three subcutaneous treatment regimens in adult subjects with uncontrolled, moderate-to-severe, persistent asthma (ClinicalTrials.gov Identifier: NCT00873860).
Lebrikizumab is a humanized monoclonal antibody that binds to IL-13 and is currently in development for asthma (ClinicalTrials.gov Identifier: NCT00930163). A phase II clinical trial evaluating the effects of this antibody in patients with asthma is ongoing However, mono-specific anti-IL-13 strategies have been disappointing in recently reported trials despite IL-13's putative importance in asthma pathogenesis. It is unclear if there is a problem with the specific monoclonal antibodies that have been developed or if there is too much redundancy in the immune system such that inhibiting either IL-4 or IL-13 is insufficient. Indeed, strategies aimed at both IL-4 and IL-13 may be a better option.
A monoclonal antibody aimed at interleukin-9 is in early phase clinical trials. However, no published data are available to ascertain its potential efficacy and safety.
Suplatast tosilate is an oral agent that has been shown to inhibit the synthesis of both IL-4 and IL-5.11 Suplatast has been shown in early clinical trials to decrease serum IgE and peripheral blood eosinophil counts.12 Suplatast has also been shown to improve traditional asthma outcomes and decrease airway inflammation.13 However, this molecule has to be used in a three times per day regimen making its utility somewhat problematic since compliance would clearly be an issue.
Considering the prominence of eosinophils in many patients with asthma and their putative role in the pathogenesis of allergic respiratory disorders, several therapeutic approaches have been developed to block eosinophil-mediated effects. Initial strategies have involved using humanized monoclonal antibodies against interleukin-5, an important cytokine for both chemotaxis and differentiation of eosinophils. Several older studies have confirmed reductions in blood and sputum eosinophils without significant changes in airway hyperresponsiveness, lung functions or symptoms in patients treated with anti-Il-5 monoclonal antibodies. Two recent studies investigated different aspects of mepolizumab therapy in patients with refractory asthma and sputum eosinophilia.14,15 Unlike previous studies, in order to enroll in these two studies, subjects had to have a high sputum eosinophil count, greater than 3%. The investigators once again found a reduction in peripheral blood eosinophil counts. However, clinical effects were modest with either no or little effects on FEV1, airway hyperresponsiveness or symptoms. There were however significant reductions in asthma exacerbations. Nonetheless, it is unclear how useful this strategy will be given that in these two studies, less than 5% of uncontrolled asthma patients met the entry criteria of having greater than 3% sputum eosinophils. Thus, the treatment appears effective for a small segment of uncontrolled asthma patients, and even in those subjects, therapeutic effectiveness was somewhat modest.
TPIASM8 is an oligonucleotide using RNA silencing. It contains two modified phosphorothioate anti-sense oligonucleotides designed to inhibit allergic inflammation by down-regulating human CCR3 and the common beta-chain of IL-3, IL-5 and GM CSF. A recent study examined the effects of inhaled TPIASM8 on sputum cellular influx, CCR3 and beta-chain mRNA in protein levels and airway physiologic responses after inhaled allergen challenge.16 Compared with placebo, TPIASM8 inhibited sputum eosinophil influx by 46% and blunted the increase in total cells after allergen challenge. TPIASM8 significantly reduced the early asthmatic response, but not the late asthmatic response. The allergen-induced levels of beta-chain mRNA and CCR3 mRNA in sputum-derived cells were inhibited by TPIASM8, but no significant effects on cell surface protein expression of CCR3 and the beta-chain were found. Larger scale clinical trials are necessary to discern the effectiveness of this treatment for asthma.
A humanized monoclonal antibody against GM CSF is also undergoing very early clinical trials. However, no data have been published to date.
TNF-α has a number of effects that could be important in asthma. TNF-α can increase airway hyperresponsiveness and increase the expression of key adhesion molecules important for eosinophil and neutrophil chemotaxis. Moreover, TNF-α can stimulate both epithelial and endothelial cells to synthesize and release chemokines and other cytokines. Several small studies in patients with severe asthma have shown that patients treated with TNF-α blockers have some improvements in lung function, quality of life, exacerbation rates and airway hyperresponsiveness. However, larger multi-site studies have been disappointing. Not only has efficacy been difficult to show, but increased adverse events were recorded indicating a relatively poor risk to benefit ratio.17 Thus, anti-TNF strategies have been placed on hold for further development in the management of asthma and allergic disorders.
An attractive target for effectively managing allergic inflammation is the mast cell. One target in the mast cell that is critical for degranulation is Syk kinase. Syk kinase is an intracellular protein tyrosine kinase that is important in mast cell and basophil activation and mediator release. Upon cross-linking of IgE bound high affinity IgE receptors, Syk kinase is activated and leads to degranulation, cytokine synthesis and leukotriene synthesis. Two antagonists, R112 and R343, have been tried in human studies. R112 demonstrated statistically significant efficacy in ameliorating symptoms of allergic rhinitis including nasal congestion, nasal discharge, sneezing, nasal and throat pruritus, post nasal drip, cough, headache and facial pain in a two-day seasonal allergic rhinitis park study setting.18 The onset of action was observed within 30 to 90 minutes and the overall clinical improvement over placebo was approximately 25%. An inhaled formulation, R343, is currently being developed for the management of allergic asthma.19
Omalizumab, the humanized monoclonal anti-IgE antibody, has been shown to have a number of anti-inflammatory and clinical benefits in patients.19,20 In patients on inhaled corticosteroids alone or in combination with other agents, the addition of omalizumab has been shown to reduce asthma exacerbations, improve symptom scores, reduce the need for inhaled and oral corticosteroids and decrease the necessity of rescue medication.21-24 Efficacy has also been demonstrated in patients who have concomitant allergic rhinitis and asthma.20
Omalizumab has been shown in several small studies or case reports to have some benefits in the following disorders: seasonal and perennial allergic rhinitis with and without asthma; atopic dermatitis; food allergies; insect allergy; chronic urticaria with auto-antibodies to either the high affinity IgE receptor or IgE itself; allergic broncho-pulmonary aspergillosis; latex allergy; chronic hyperplastic sinusitis; recurrent nasal polyposis; drug allergy; and idiopathic anaphylaxis.21
Omalizumab decreases free IgE levels rapidly and the expression of the high affinity IgE receptor expression on key effector cells, including mast cells, basophils, dendritic cells and monocytes. In addition, lung inflammation has been shown to decrease after only sixteen weeks of omalizumab treatment. However, omalizumab has been reported to cause anaphylaxis through unknown mechanisms in first as well as subsequent doses.22
Observation of patients for 2 hours after they received each of the first three injections and for 30 minutes after they received subsequent injections would capture 75% of the anaphylactic reactions according to the current recommendation of the American Academy of Allergy, Asthma & Immunology/American College of Allergy, Asthma & Immunology Joint Task Force.23
There have been many attempts to develop antagonists to mast cell mediators that play a significant role in airway hyperreactivity in asthma. Antagonists to CRTH2 (a G protein coupled receptor) are currently under investigation. CRTH2 is present on Th2-cells, eosinophils and basophils mediating activation and chemotaxis to PGD2. A recent 28-day phase II study in mild to moderate asthma patients with FEV1 values between 60 and 80% showed promising trends with ACT-129968, an oral antagonist.24 Bronchoprovocation to both adenosine monophosphate and methacholine decreased by one to one and a half doubling doses, FEV1 increased by 10 to 14%, and there was a decrease in sputum eosinophils and IgE. Moreover, patients had an improved quality of life. Further studies examining the efficacy of this approach will be necessary to determine true therapeutic potential.
There are a number of immunomodulators that have been developed for the treatment of allergic disorders. Some have looked very promising in pre-clinical trials, but have not shown significant benefits in human clinical trials indicating the disparity between murine models and human asthma.
It is difficult to predict which of these pharmaceutical agents other than omalizumab will achieve success. Ultimately, the use of these putative therapeutics in human clinical trials should help define the pathogenesis of asthma and determine the importance of specific molecules in large patient populations. It is possible that some of these agents could be effective for only a small sub-population of patients which potentially would make them unlikely to be commercially viable.
The search for better therapeutics options is focused on decreasing the symptoms and improving the quality of life while preventing or altering disease course. These molecules should also have a favorable risk benefit ratio which would lead to wider use. In addition, cost effective strategies are clearly indicated as monoclonal antibodies are very expensive to manufacture and to administer.
The use of immunomodulators for the treatment of allergic respiratory disorders represents an exciting new era for the therapy of allergic disorders. Human clinical trials are essential for the development and to determine their ultimate place in our therapeutic armamentarium.
Figures and Tables
References
1. Malmstrom K, Rodriguez-Gomez G, Guerra J, Villaran C, Pineiro A, Wei LX, Seidenberg BC, Reiss TF. Montelukast/Beclomethasone Study Group. Oral montelukast, inhaled beclomethasone, and placebo for chronic asthma. A randomized, controlled trial. Ann Intern Med. 1999. 130:487–495.
2. Tantisira KG, Lake S, Silverman ES, Palmer LJ, Lazarus R, Silverman EK, Liggett SB, Gelfand EW, Rosenwasser LJ, Richter B, Israel E, Wechsler M, Gabriel S, Altshuler D, Lander E, Drazen J, Weiss ST. Corticosteroid pharmacogenetics: association of sequence variants in CRHR1 with improved lung function in asthmatics treated with inhaled corticosteroids. Hum Mol Genet. 2004. 13:1353–1359.
3. Busse WW, Israel E, Nelson HS, Baker JW, Charous BL, Young DY, Vexler V, Shames RS. Daclizumab improves asthma control in patients with moderate to severe persistent asthma: a randomized, controlled trial. Am J Respir Crit Care Med. 2008. 178:1002–1008.
4. Salapatek AM, Patel P, Schulze J, Fischer von Weikersthal-Drachenberg KJ, Zielen S. Comparison of outcomes following ultra short course specific immunotherapy (uSCIT) in juvenile and adult patients with asthma. Proceedings of the XXVIIth Congress of the European Academy of Allergology and Clinical Immunology (EAACI). 2008. Jun 7-11; Barcelona, Spain.
5. Creticos PS, Schroeder JT, Hamilton RG, Balcer-Whaley SL, Khattignavong AP, Lindblad R, Li H, Coffman R, Seyfert V, Eiden JJ, Broide D. Immunotherapy with a ragweed-toll-like receptor 9 agonist vaccine for allergic rhinitis. N Engl J Med. 2006. 355:1445–1455.
6. Senti G, Johansen P, Haug S, Bull C, Gottschaller C, Müller P, Pfister T, Maurer P, Bachmann MF, Graf N, Kündig TM. Use of A-type CpG oligodeoxynucleotides as an adjuvant in allergen-specific immunotherapy in humans: a phase I/IIa clinical trial. Clin Exp Allergy. 2009. 39:562–570.
7. Blaziene A. CYT003-QbG10, a novel allergen-independent immunotherapy, shown to be safe and efficacious in placebo-controlled phase II study. Proceedings of the American College of Allergy, Asthma & Immunology Annual Scientific Meeting. 2008. Nov 6-11; Seattle, USA.
8. Belvisi MG, Hele DJ. Peroxisome proliferator-activated receptors as novel targets in lung disease. Chest. 2008. 134:152–157.
9. Corren J, Busse W, Meltzer E, Mansfield L, Bensch G, Chon Y, Dunn M, Weng H, Lin S. Efficacy and safety of AMG 317, an IL-4Ra antagonist, in atopic asthmatic subjects: a randomized, double-blind, placebo-controlled study. J Allergy Clin Immunol. 2009. 123:732.
10. Wenzel S, Wilbraham D, Fuller R, Getz EB, Longphre M. Effect of an interleukin-4 variant on late phase asthmatic response to allergen challenge in asthmatic patients: results of two phase 2a studies. Lancet. 2007. 370:1422–1431.
11. Ishiura Y, Fujimura M, Yamamoto H, Nobata K, Ishiguro T, Ogawa H, Myou S. Effect of an orally active Th2 cytokine inhibitor, suplatast on "atopic cough" tosilate. Arzneimittelforschung. 2008. 58:297–302.
12. Agrawal DK, Cheng G, Kim MJ, Kiniwa M. Interaction of suplatast tosilate (IPD) with chloride channels in human blood eosinophils: a potential mechanism underlying its anti-allergic and anti-asthmatic effects. Clin Exp Allergy. 2008. 38:305–312.
13. Tanaka A, Minoguchi K, Samson KT, Oda N, Yokoe T, Tazaki T, Yamamoto Y, Yamamoto M, Ohta S, Adachi M. Inhibitory effects of suplatast tosilate on the differentiation and function of monocyte-derived dendritic cells from patients with asthma. Clin Exp Allergy. 2007. 37:1083–1089.
14. Haldar P, Brightling CE, Hargadon B, Gupta S, Monteiro W, Sousa A, Marshall RP, Bradding P, Green RH, Wardlaw AJ, Pavord ID. Mepolizumab and exacerbations of refractory eosinophilic asthma. N Engl J Med. 2009. 360:973–984.
15. Nair P, Pizzichini MM, Kjarsgaard M, Inman MD, Efthimiadis A, Pizzichini E, Hargreave FE, O'Byrne PM. Mepolizumab for prednisone-dependent asthma with sputum eosinophilia. N Engl J Med. 2009. 360:985–993.
16. Gauvreau GM, Boulet LP, Cockcroft DW, Baatjes A, Cote J, Deschesnes F, Davis B, Strinich T, Howie K, Duong M, Watson RM, Renzi PM, O'Byrne PM. Antisense therapy against CCR3 and the common beta chain attenuates allergen-induced eosinophilic responses. Am J Respir Crit Care Med. 2008. 177:952–958.
17. Wenzel SE, Barnes PJ, Bleecker ER, Bousquet J, Busse W, Dahlen SE, Holgate ST, Meyers DA, Rabe KF, Antczak A, Baker J, Horvath I, Mark Z, Bernstein D, Kerwin E, Schlenker-Herceg R, Lo KH, Watt R, Barnathan ES, Chanez P. A randomized, double-blind, placebo-controlled study of tumor necrosis factor-alpha blockade in severe persistent asthma. Am J Respir Crit Care Med. 2009. 179:549–558.
18. Meltzer EO, Berkowitz RB, Grossbard EB. An intranasal Syk-kinase inhibitor (R112) improves the symptoms of seasonal allergic rhinitis in a park environment. J Allergy Clin Immunol. 2005. 115:791–796.
19. Rigel announces initiation of phase 1 clinical trial of R343 for allergic asthma by its partner Pfizer [Internt]. Rigel Pharmaceuticals, Inc. cited 2009 Jun 12. Available from: http://ir.rigel.com/phoenix.zhtml?c=120936&p=irol-newsArticle&ID=1084738.
20. Casale TB, Condemi J, LaForce C, Nayak A, Rowe M, Watrous M, McAlary M, Fowler-Taylor A, Racine A, Gupta N, Fick R, Della Cioppa G. Effect of omalizumab on symptoms of seasonal allergic rhinitis: a randomized controlled trial. JAMA. 2001. 286:2956–2967.
21. Casale TB, Stokes J. Anti-IgE therapy: clinical utility beyond asthma. J Allergy Clin Immunol. 2009. 123:770–771.e1.
22. Limb SL, Starke PR, Lee CE, Chowdhury BA. Delayed onset and protracted progression of anaphylaxis after omalizumab administration in patients with asthma. J Allergy Clin Immunol. 2007. 120:1378–1381.
23. Cox L, Platts-Mills TA, Finegold I, Schwartz LB, Simons FE, Wallace DV. American Academy of Allergy, Asthma & Immunology/American College of Allergy, Asthma and Immunology Joint Task Force Report on omalizumab-associated anaphylaxis. J Allergy Clin Immunol. 2007. 120:1373–1377.
24. CRTH2 antagonist [Internet]. Actelion Pharmaceuticals Ltd. cited 2010 Feb 24. Available from: http://www.actelion.com/en/scientists/development-pipeline/phase-2/crth2-antagonist.page.
25. Casale TB, Stokes JR. Immunomodulators for allergic respiratory disorders. J Allergy Clin Immunol. 2008. 121:288–296. quiz 97-8.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download