Abstract
Concept of asthma has changed from symptom-complex or airway hypersensitivity to airway inflammation and airway remodeling. Based on this concept asthma management guidelines (JGL) has been developed in Japan. Death from asthma has decreased drastically since the publication of the guidelines, although it is still high in elderly population. Further works are expected for "zero-death" from asthma and for tighter control of airway inflammation and resultant airway remodeling.
Asthma is a chronic inflammatory disease of the airway associated with airway hyperresponsiveness and episodes of airway narrowing. Facts of asthma in WHO website in 2010 said;
in 2005 in the world.1
Treatment of asthma progressed aligned with evolution of concept of asthma.2 In the middle part of 20th century asthma was called as a disease or symptom complex which showed paroxysmal dyspnea with reversible bronchoconstriction. Spasm of bronchial smooth muscle was thought to be major mechanism of airway narrowing.3 Accordingly, bronchodilators including epinephrine, isoproterenol and theophyllines were major agents to treat asthma attacks. In 1960s asthma was defined as disease of airway hyperresponsiveness.4,5 Around 1980s, inflammation of the airway was found to be related with airway hyperresponsiveness and asthma symptoms. Eosinophils are most outstanding cells in the airway of asthma patients. Eosinophils are found to cause or contribute to histological and functional changes in the airway of asthma patients including desquamation of bronchial epithelium and airway hyperresponsiveness.6-8 Anti-inflammatory agents including corticosteroids, anti-allergic agents, theophyllines, are used to suppress eosinophilia in the airway and has been proved to be effective.9,10 Following studies on pathophysiology of asthma have shown that many cells and cytokines have roles in the inflammation of the airway of asthma patients in complicated manner.11 Structural change of the airway due to airway inflammation (airway remodeling) has been shown to contribute to persistent airway narrowing and airway hyperresponsiveness (Fig. 1).12
The basic pathological features of bronchial asthma can be explained on the basis of chronic airway inflammation, involving inflammatory cells such as T cells (particularly type 2 helper T, Th2 cells) and mast cells, and airway remodeling. Airway remodeling causes persistent airway narrowing and airway hyperresponsiveness.12 In fact back to 1966 Makino found that asthma patients with low FEV1/Predicted VC had increased airway responsiveness.13
Recent attention has focused on the role of transforming growth factor (TGF)-beta, a fibrogenic cytokine, in airway remodeling. Currently available evidence suggests that airway remodeling is caused by an imbalance in regulatory mechanisms mediated by Smads, a family of signal-transducing molecules of TGF-beta. Smad7 is an intracellular antagonist of TGF-beta signaling, which could determine the intensity or duration of the TGF-beta signal. Sagara, Nakao and others found that the expression of Smad7 in bronchial epithelial cells is inversely correlated to basement membrane thickness and airway hyperresponsiveness in patients with asthma, while the expression of phosphorylated activated Smad2 (p-Smad2) is positively correlated with them.14,15 Intracellular level of Smad protein is controlled by cytokines. Fueki, Sagara and other found the effects of the Th2 cytokines interleukin (IL)-5 and granulocyte-macrophage colony-stimulating factor (GM-CSF), and the regulatory cytokine IL-10 on the expression of inhibitory Smad7 protein in bronchial epithelial cells IL-10 inhibited the expression of TGF-beta-inducible early gene, which is known to down-regulate Smad7 expression.16 McMillan and others found budesonide treatment regulated active TGF-beta signaling with a reduction in the expression of Smad2 and the concomitant up-regulation of Smad7 in lung tissue sections of experimental asthma of mice.17
In 1992 International Consensus Report for the Diagnosis and Management of asthma was released.18 Global Initiative for Asthma (GINA) started in 1993 and was published in 1995. GINA was developed on the concept of airway inflammation of the airway.19
In Japan of 1993, Asthma Prevention and Management Guidelines (JGL) was first developed by Japanese Society of Allergology.20-22 Like GINA JGL has been produced with the concept that asthma is an inflammatory disease of the airway, and its treatment is aimed at prevention and control of airway inflammation. This concept is new and different from the previous understanding of asthma. Since then JGL has been revised in 1997, 2002, 2006, and 2009, and accepted as the standard of asthma management in Japan.23
Effectiveness of asthma management guidelines can be evaluated by the change of death from asthma comparing before and after implementation of JGL, and trend of medical expenses for the treatment of asthma. The total number of death from asthma for each year was obtained by the report of Ministry of Health, Welfare and Labor, Japan.24
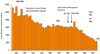
Death from asthma seems to have started to decrease continuously around 1997 to 2008. The total number of death from asthma from 1993 to 1998 was approximately 5900, and it was 2300 in 2008. JGL has been widely known in both specialists and non-specialists and widely accepted. Increased use of anti-inflammatory agents including inhaled corticosteroids is thought to be one of causes of this decrease (Fig. 3).20,23
GINA has been accepted as a template of national asthma management guidelines in many of countries in Asia Pacific. Implementation of asthma management guidelines with concept that asthma is an inflammatory disease of airway would be effective to decrease of death from asthma in both developed and developing countries.
Another important issue on asthma control is its expense. While in Japan the death from asthma has decreased drastically for the past one decade, direct medical expense for asthma treatment has not increased.24 This finding suggests that proper implementation of asthma management guidelines is not expensive and would contribute to the social productivity by decreasing work loss and absence for school.
Death from asthma has decreased in children to younger adult populations, while it is still high in elderly population especially 65 years and over. This can be partly due to increase of elderly population at larger. In fact population 65 years and over is approximately 23% of total Japanese population in 2009. Apparently, control of death from asthma in elderly population is another target of Japanese Guidelines.25
Figures and Tables
 | Fig. 1Evolution of Asthma Concept from Makino S.2
ATS, American Thoracic Society; ICR, International Consensus Report; JGL, Japanese Asthma Prevention and Management Guidelines; GINA, Global Initiative for Asthma.
|
 | Fig. 2-1Smad7 seems to suppress thickening of bronchial basement membrane in asthma.15 MB: Basement membrane of the epithelium. |
 | Fig. 2-2p-Smad2 expression level in bronchial epithelial cells was elevated in asthma. Basement membrane thickness was correlated with Smad2 expression level in the airway of mild to severe asthmatics.14
|
References
1. Asthma. Quick asthma facts & The Faces of asthma [Internet]. 2010. Geneva: World Health Organization;Available from:
http://www.who.int/respiratory/asthma/en/.
2. Makino S. History of asthma treatment. Zensoku (Asthma). 2005. 18:17–21. Japanese.
3. Solomon WR. Sheldon JM, Lovell RC, Mathews KP, editors. Hay fever. Allergic rhinitis and bronchial asthma. A Manual of Clinical Allergy. 1967. Philadelphia: W.B. Saunders Co.;78–97.
4. American Thoracic Society. Chronic bronchitis, asthma, and pulmonary emphysema: a statement by the Committee on Diagnostic Standards for Nontuberculous Respiratory Diseases. Am Rev Respir Dis. 1962. 85:762–768.
5. ACCP-ATS Joint Committee on Pulmonary Nomenclature. Pulmonary terms and symbols. Chest. 1975. 67:583–593.
6. Gleich GJ. The eosinophil and bronchial asthma: current understanding. J Allergy Clin Immunol. 1990. 85:422–436.
7. Makino S, Fukuda T, editors. Eosinophils: biological and clinical aspects. 1993. Boca Raton (FL): CRC Press.
8. Berman JS, Weller PF. Airway eosinophils and lymphocytes in asthma. Birds of a feather? Am Rev Respir Dis. 1992. 145:1246–1248.
9. Church MK, Makino S. Holgate ST, Church MK, Lichenstein LM, editors. Drugs for the treatment of allergic disease. Allergy. 2006. 3rd ed. Philadelphia (PA): Mosby Elsevier;353–370.
10. Makino S, Adachi M, Ohta K, Kihara N, Nakajima S, Nishima S, Fukuda T, Miyamoto T. A prospective survey on safety of sustained-release theophylline in treatment of asthma and COPD. Allergol Int. 2006. 55:395–402.
11. Barnes PJ. The cytokine network in asthma and chronic obstructive pulmonary disease. J Clin Invest. 2008. 118:3546–3556.
12. Sumi Y, Hamid Q. Airway remodeling in asthma. Allergol Int. 2007. 56:341–348.
13. Makino S. Clinical significance of bronchial sensitivity to acetylcholine and histamine in bronchial asthma. J Allergy. 1966. 38:127–142.
14. Sagara H, Okada T, Okumura K, Ogawa H, Ra C, Fukuda T, Nakao A. Activation of TGF-beta/Smad2 signaling is associated with airway remodeling in asthma. J Allergy Clin Immunol. 2002. 110:249–254.
15. Nakao A, Sagara H, Setoguchi Y, Okada T, Okumura K, Ogawa H, Fukuda T. Expression of Smad7 in bronchial epithelial cells is inversely correlated to basement membrane thickness and airway hyperresponsiveness in patients with asthma. J Allergy Clin Immunol. 2002. 110:873–878.
16. Fueki N, Sagara H, Akimoto K, Ota M, Okada T, Sugiyama K, Fueki M, Makino S, Fukuda T. Interleukin-10 regulates transforming growth factor-beta signaling in cultured human bronchial epithelial cells. Respiration. 2007. 74:454–459.
17. McMillan SJ, Xanthou G, Lloyd CM. Therapeutic administration of Budesonide ameliorates allergen-induced airway remodelling. Clin Exp Allergy. 2005. 35:388–396.
18. International Consensus Report on Diagnosis and Management of Asthma. International Asthma Management Project. Allergy. 1992. 47:13 Suppl. 1–61.
19. Global Strategy for Asthma Management and Prevention. National Institutes of Health, 1995. NIH publication no. 02-3659. Global Initiative for Asthma. Available from:
http://www.ginasthma.org/GuidelineItem.asp?intId=82.
20. Makino S, Furusho K, Miyamoto T, Ohta K, editors. [Research Group for Asthma Prevention and Management Guidelines, supported by the Ministry of Health and Welfare, Japan. Asthma prevention and management guidelines, Japan (JGL1993)]. 1993. Japanese.
21. Makino S, Adachi M, Ago Y, Akiyama K, Baba M, Egashira Y, Fujimura M, Fukuda T, Furusho K, Iikura Y, Inoue H, Ito K, Iwamoto I, Kabe J, Kamikawa Y, Kawakami Y, Kihara N, Kitamura S, Kudo K, Mano K, Matsui T, Mikawa H, Miyagi S, Miyamoto T, Morita Y, Nagasaka Y, Nakagawa T, Nakajima S, Nakazawa T, Nishima S, Ohta K, Okubo T, Sakakibara H, Sano Y, Shinomiya K, Takagi K, Takahashi K, Tamura G, Tomioka H, Yoyoshima K, Tsukioka K, Ueda N, Yamakido M, Hosoi S, Sagara H. Pharmacologic control of asthma. Int Arch Allergy Immunol. 2005. 136:Suppl 1. 14–49.
22. Makino S, Adachi M, Ago Y, Akiyama K, Baba M, Egashira Y, Fujimura M, Fukuda T, Furusho K, Iikura Y, Inoue H, Ito K, Iwamoto I, Kabe J, Kamikawa Y, Kawakami Y, Kihara N, Kitamura S, Kudo K, Mano K, Matsui T, Mikawa H, Miyagi S, Miyamoto T, Morita Y, Nagasaka Y, Nakagawa T, Nakajima S, Nakazawa T, Nishima S, Ohta K, Okubo T, Sakakibara H, Sano Y, Shinomiya K, Takagi K, Takahashi K, Tamura G, Tomioka H, Yoyoshima K, Tsukioka K, Ueda N, Yamakido M, Hosoi S, Sagara H. Epidemiology of asthma. Int Arch Allergy Immunol. 2005. 136:Suppl 1. 5–13.
23. Makino S, Miyamoto T, Nakajima S, Kabe J, Baba M, Mikawa H, Furusho M, Fukuda K, Nakagawa T, Naitou H. Survey of recognition and utilization of guidelines for the diagnosis and management of bronchial asthma in Japan. Allergy. 2000. 55:135–140.
24. Ministry of Health, Labor and Welfare, Japan [Internet]. Available from:
http://www.mhlw.go.jp/english/index.html. Japanese.
25. Japanese Society of Allergology, Asthma Guideline Committee. [Asthma Prevention and Management Guidelines 2006]. 2006. Tokyo: Kyowa Kikaku;Japanese.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download