1. Kim YY, Holgate ST, Church MK. Inhibition of histamine release from dispersed human lung and tonsillar mast cells by nicardipine and nifedipine. Br J Clin Pharmacol. 1985; 19:631–638. PMID:
2408645.

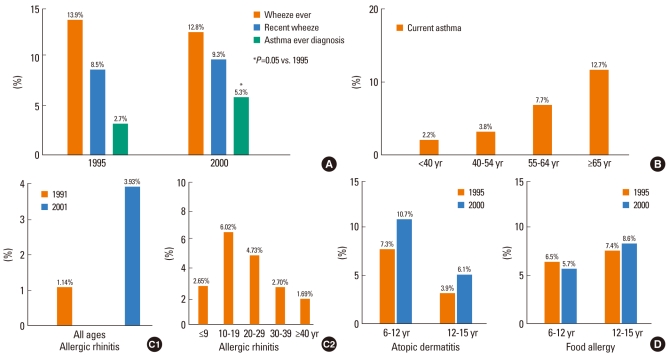
2. Cho SH, Park HW, Rosenberg DM. The current status of asthma in Korea. J Korean Med Sci. 2006; 21:181–187. PMID:
16614498.

3. Hong SJ, Lee MS, Sohn MH, Shim JY, Han YS, Park KS, Ahn YM, Son BK, Lee HB. Self-reported prevalence and risk factors of asthma among Korean adolescents: 5-year follow-up study, 1995-2000. Clin Exp Allergy. 2004; 34:1556–1562. PMID:
15479270.

4. Kim YY, Cho SH, Kim WK, Park JK, Song SH, Kim YK, Jee YK, Ha MN, Ahn YO, Lee SI, Min KU. Prevalence of childhood asthma based on questionnaires and methacholine bronchial provocation test in Korea. Clin Exp Allergy. 1997; 27:761–768. PMID:
9249268.

5. Kim YK, Kim SH, Tak YJ, Jee YK, Lee BJ, Park HW, Jung JW, Bahn JW, Chang YS, Choi DC, Chang SI, Min KU, Kim YY, Cho SH. High prevalence of current asthma and active smoking effect among the elderly. Clin Exp Allergy. 2002; 32:1706–1712. PMID:
12653160.

6. Min YG, Choi BY, Kwon SK, Lee SS, Jung YH, Kim JW, Oh SJ. Multicenter study on the prevalence of perennial allergic rhinitis and allergy-associated disorders. J Korean Med Sci. 2001; 16:697–701. PMID:
11748347.

7. Oh JW, Pyun BY, Choung JT, Ahn KM, Kim CH, Song SW, Son JA, Lee SY, Lee SI. Epidemiological change of atopic dermatitis and food allergy in school-aged children in Korea between 1995 and 2000. J Korean Med Sci. 2004; 19:716–723. PMID:
15483350.

8. Kim TB, Kim KM, Kim SH, Kang HR, Chang YS, Kim CW, Bahn JW, Kim YK, Kang HT, Cho SH, Park HS, Lee JM, Choi IS, Min KU, Hong CS, Kim NS, Kim YY. Sensitization rates for inhalant allergens in Korea; a multi-center study. J Asthma Allergy Clin Immunol. 2003; 23:483–493. Korean.
9. Kim YK, Kim YY. Spider-mite allergy and asthma in fruit growers. Curr Opin Allergy Clin Immunol. 2002; 2:103–107. PMID:
11964757.

10. Kim YK, Lee MH, Jee YK, Hong SC, Bae JM, Chang YS, Jung JW, Lee BJ, Son JW, Cho SH, Min KU, Kim YY. Spider mite allergy in apple-cultivating farmers: European red mite (Panonychus ulmi) and two-spotted spider mite (Tetranychus urticae) may be important allergens in the development of work-related asthma and rhinitis symptoms. J Allergy Clin Immunol. 1999; 104:1285–1292. PMID:
10589014.

11. Kim YK, Son JW, Kim HY, Park HS, Lee MH, Cho SH, Min KU, Kim YY. New occupational allergen in citrus farmers: citrus red mite (Panonychus citri). Ann Allergy Asthma Immunol. 1999; 82:223–228. PMID:
10071529.

12. Kim YK, Son JW, Kim HY, Park HS, Lee MH, Cho SH, Min KU, Kim YY. Citrus red mite (Panonychus citri) is the most common sensitizing allergen of asthma and rhinitis in citrus farmers. Clin Exp Allergy. 1999; 29:1102–1109. PMID:
10457115.

13. Kim YK, Park HS, Kim HY, Jee YK, Son JW, Bae JM, Lee MH, Cho SH, Min KU, Kim YY. Citrus red mite (Panonychus citri) may be an important allergen in the development of asthma among exposed children. Clin Exp Allergy. 2001; 31:582–589. PMID:
11359426.

14. Kim TB, Kim YK, Chang YS, Kim SH, Hong SC, Jee YK, Cho SH, Min KU, Kim YY. Association between sensitization to outdoor spider mites and clinical manifestations of asthma and rhinitis in the general population of adults. J Korean Med Sci. 2006; 21:247–252. PMID:
16614509.

15. Park HS, Kim HY, Nahm DH, Son JW, Kim YY. Specific IgG, but not specific IgE, antibodies to toluene diisocyanate-human serum albumin conjugate are associated with toluene diisocyanate bronchoprovocation test results. J Allergy Clin Immunol. 1999; 104:847–851. PMID:
10518831.

16. Park HS, Jung KS, Kim HY, Nahm DH, Kang KR. Neutrophil activation following TDI bronchial challenges to the airway secretion from subjects with TDI-induced asthma. Clin Exp Allergy. 1999; 29:1395–1401. PMID:
10520061.

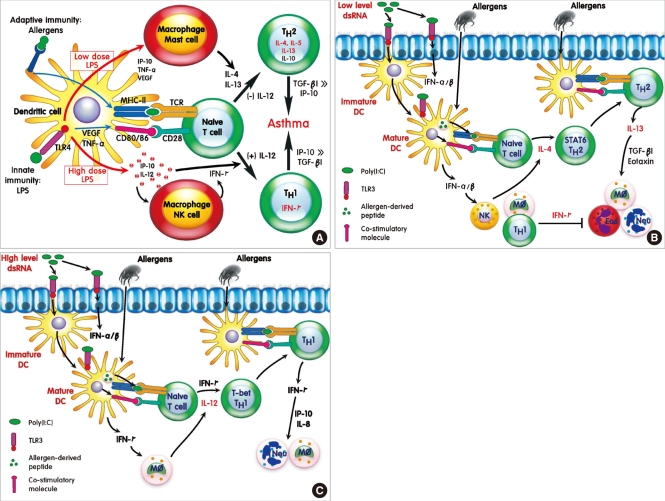
17. Park HS, Cho SH, Hong CS, Kim YY. Isocyanate-induced occupational asthma in far-east Asia: pathogenesis to prognosis. Clin Exp Allergy. 2002; 32:198–204. PMID:
11929482.

18. Ye YM, Kim CW, Kim HR, Kim HM, Suh CH, Nahm DH, Park HS, Redlich CA, Wisnewski AV. Biophysical determinants of toluene diisocyanate antigenicity associated with exposure and asthma. J Allergy Clin Immunol. 2006; 118:885–891. PMID:
17030242.

19. Park HS, Kim YJ, Lee MK, Hong CS. Occupational asthma and IgE antibodies to reactive dyes. Yonsei Med J. 1989; 30:298–304. PMID:
2588667.

20. Park HS, Kim JW, Hong CS. The prevalence of specific IgE and IgG to reactive dye-human serum albumin conjugate in workers of a dye factory and neighboring factories. J Korean Med Sci. 1991; 6:63–68. PMID:
1888451.

21. Lee SK, Cho HK, Cho SH, Kim SS, Nahm DH, Park HS. Occupational asthma and rhinitis caused by multiple herbal agents in a pharmacist. Ann Allergy Asthma Immunol. 2001; 86:469–474. PMID:
11345295.

22. Lee JY, Lee YD, Bahn JW, Park HS. A case of occupational asthma and rhinitis caused by Sanyak and Korean ginseng dusts. Allergy. 2006; 61:392–393. PMID:
16436153.

23. Hur GY, Park HJ, Kim HA, Ye YM, Park HS. Identification of Dioscorea batatas (sanyak) allergen as an inhalant and oral allergen. J Korean Med Sci. 2008; 23:72–76. PMID:
18303202.
24. Kim KM, Kwon HS, Jeon SG, Park CH, Sohn SW, Kim DI, Kim SS, Chang YS, Kim YK, Cho SH, Min KU, Kim YY. Korean ginseng-induced occupational asthma and determination of IgE binding components. J Korean Med Sci. 2008; 23:232–235. PMID:
18437005.

25. Nahm DH, Park JW, Hong CS. Occupational asthma due to deer dander. Ann Allergy Asthma Immunol. 1996; 76:423–426. PMID:
8630715.

26. Kim WH, Lee SK, Lee HC, Hong CS, Huh KB, Lee WY, Lee SY. Shellgrinder's asthma. Yonsei Med J. 1982; 23:123–130. PMID:
7187148.

27. Cho SH, Seo JY, Choi DC, Yoon HJ, Cho YJ, Min KU, Lee GK, Seo JW, Kim YY. Pathological changes according to the severity of asthma. Clin Exp Allergy. 1996; 26:1210–1219. PMID:
8911709.

28. Park CS, Choi YS, Ki SY, Moon SH, Jeong SW, Uh ST, Kim YH. Granulocyte macrophage colony-stimulating factor is the main cytokine enhancing survival of eosinophils in asthmatic airways. Eur Respir J. 1998; 12:872–878. PMID:
9817161.

29. Chang HS, Jeon KW, Kim YH, Chung IY, Park CS. Role of cAMP-dependent pathway in eosinophil apoptosis and survival. Cell Immunol. 2000; 203:29–38. PMID:
10915559.

30. Chung IY, Nam-Kung EK, Lee NM, Chang HS, Kim DJ, Kim YH, Park CS. The downregulation of Bcl-2 expression is necessary for theophylline-induced apoptosis of eosinophil. Cell Immunol. 2000; 203:95–102. PMID:
11006007.

31. Kim YK, Oh SY, Jeon SG, Park HW, Lee SY, Chun EY, Bang B, Lee HS, Oh MH, Kim YS, Kim JH, Gho YS, Cho SH, Min KU, Kim YY, Zhu Z. Airway exposure levels of lipopolysaccharide determine type 1 versus type 2 experimental asthma. J Immunol. 2007; 178:5375–5382. PMID:
17404323.

32. Jeon SG, Oh SY, Park HK, Kim YS, Shim EJ, Lee HS, Oh MH, Bang B, Chun EY, Kim SH, Gho YS, Zhu Z, Kim YY, Kim YK. TH2 and TH1 lung inflammation induced by airway allergen sensitization with low and high doses of double-stranded RNA. J Allergy Clin Immunol. 2007; 120:803–812. PMID:
17610940.

33. Chang YS, Kim YK, Bahn JW, Kim SH, Park HW, Kim TB, Cho SH, Min KU, Kim YY. Comparison of asthma phenotypes using different sensitizing protocols in mice. Korean J Intern Med. 2005; 20:152–158. PMID:
16134771.

34. Kim YK, Baek D, Koh YI, Cho SH, Choi IS, Min KU, Kim YY. Outdoor air pollutants derived from industrial processes may be causally related to the development of asthma in children. Ann Allergy Asthma Immunol. 2001; 86:456–460. PMID:
11345292.

35. Park JK, Kim YK, Lee SR, Cho SH, Min KU, Kim YY. Repeated exposure to low levels of sulfur dioxide (SO2) enhances the development of ovalbumin-induced asthmatic reactions in guinea pigs. Ann Allergy Asthma Immunol. 2001; 86:62–67. PMID:
11206242.

36. Jang AS, Choi IS, Takizawa H, Rhim T, Lee JH, Park SW, Park CS. Additive effect of diesel exhaust particulates and ozone on airway hyperresponsiveness and inflammation in a mouse model of asthma. J Korean Med Sci. 2005; 20:759–763. PMID:
16224148.

37. Park Y, Chang YS, Lee SW, Cho SY, Kim YK, Min KU, Kim YY, Cho SH, Sung YC. The enhanced effect of a hexameric deoxyriboguanosine run conjugation to CpG oligodeoxynucleotides on protection against allergic asthma. J Allergy Clin Immunol. 2001; 108:570–576. PMID:
11590383.

38. Jeon SG, Lee CG, Oh MH, Chun EY, Gho YS, Cho SH, Kim JH, Min KU, Kim YY, Kim YK, Elias JA. Recombinant basic fibroblast growth factor inhibits the airway hyperresponsiveness, mucus production, and lung inflammation induced by an allergen challenge. J Allergy Clin Immunol. 2007; 119:831–837. PMID:
17289133.

39. Cho YS, Kim TB, Lee TH, Moon KA, Lee J, Kim YK, Lee KY, Moon HB. Chlamydia pneumoniae infection enhances cellular proliferation and reduces steroid responsiveness of human peripheral blood mononuclear cells via a tumor necrosis factor-alpha-dependent pathway. Clin Exp Allergy. 2005; 35:1625–1631. PMID:
16393329.

40. Choi JM, Ahn MH, Chae WJ, Jung YG, Park JC, Song HM, Kim YE, Shin JA, Park CS, Park JW, Park TK, Lee JH, Seo BF, Kim KD, Kim ES, Lee DH, Lee SK. Intranasal delivery of the cytoplasmic domain of CTLA-4 using a novel protein transduction domain prevents allergic inflammation. Nat Med. 2006; 12:574–579. PMID:
16604087.

41. Park HS, Kim SH, Park CS. The role of novel genes in modifying airway responses in asthma. Curr Allergy Asthma Rep. 2006; 6:112–116. PMID:
16566860.

42. Kim SH, Cho BY, Park CS, Shin ES, Cho EY, Yang EM, Kim CW, Hong CS, Lee JE, Park HS. Alpha-T-catenin (CTNNA3) gene was identified as a risk variant for toluene diisocyanate-induced asthma by genome-wide association analysis. Clin Exp Allergy. 2009; 39:203–212. PMID:
19187332.

43. Lee JH, Park HS, Park SW, Jang AS, Uh ST, Rhim T, Park CS, Hong SJ, Holgate ST, Holloway JW, Shin HD. ADAM33 polymorphism: association with bronchial hyper-responsiveness in Korean asthmatics. Clin Exp Allergy. 2004; 34:860–865. PMID:
15196271.

44. Lee JY, Park SW, Chang HK, Kim HY, Rhim T, Lee JH, Jang AS, Koh ES, Park CS. A disintegrin and metalloproteinase 33 protein in patients with asthma: Relevance to airflow limitation. Am J Respir Crit Care Med. 2006; 173:729–735. PMID:
16387804.
45. Shin HD, Park BL, Kim LH, Jung JH, Wang HJ, Kim YJ, Park HS, Hong SJ, Choi BW, Kim DJ, Park CS. Association of tumor necrosis factor polymorphisms with asthma and serum total IgE. Hum Mol Genet. 2004; 13:397–403. PMID:
14681301.

46. Park BL, Kim LH, Choi YH, Lee JH, Rhim T, Lee YM, Uh ST, Park HS, Choi BW, Hong SJ, Park CS, Shin HD. Interleukin 3 (IL3) polymorphisms associated with decreased risk of asthma and atopy. J Hum Genet. 2004; 49:517–527. PMID:
15372320.

47. Chang HS, Kim JS, Lee JH, Cho JI, Rhim TY, Uh ST, Park BL, Chung IY, Park CS, Shin HD. A single nucleotide polymorphism on the promoter of eotaxin1 associates with its mRNA expression and asthma phenotypes. J Immunol. 2005; 174:1525–1531. PMID:
15661912.

48. Cheong HS, Kim LH, Park BL, Choi YH, Park HS, Hong SJ, Choi BW, Park CS, Shin HD. Association analysis of interleukin 5 receptor alpha subunit (IL5RA) polymorphisms and asthma. J Hum Genet. 2005; 50:628–634. PMID:
16217591.

49. Cheong HS, Park CS, Kim LH, Park BL, Uh ST, Kim YH, Lym GI, Lee JY, Lee JK, Kim HT, Ryu HJ, Han BG, Kim JW, Park C, Kimm K, Shin HD, Oh B. CXCR3 polymorphisms associated with risk of asthma. Biochem Biophys Res Commun. 2005; 334:1219–1225. PMID:
16043121.

50. Park HW, Shin ES, Lee JE, Kim SH, Kim SS, Chang YS, Kim YK, Min KU, Kim YY, Cho SH. Association between genetic variations in prostaglandin E2 receptor subtype EP3 gene (Ptger3) and asthma in the Korean population. Clin Exp Allergy. 2007; 37:1609–1615. PMID:
17877755.
51. Oh SH, Park SM, Lee YH, Cha JY, Lee JY, Shin EK, Park JS, Park BL, Shin HD, Park CS. Association of peroxisome proliferator-activated receptor-gamma gene polymorphisms with the development of asthma. Respir Med. 2009; 103:1020–1024. PMID:
19217272.

52. Park HW, Lee JE, Shin ES, Lee JY, Bahn JW, Oh HB, Oh SY, Cho SH, Moon HB, Min KU, Elias JA, Kim YY, Kim YK. Association between genetic variations of vascular endothelial growth factor receptor 2 and atopy in the Korean population. J Allergy Clin Immunol. 2006; 117:774–779. PMID:
16630933.

53. Kim ES, Kim SH, Kim KW, Park HS, Shin ES, Lee JE, Sohn MH, Kim KE. Involvement of Fc(epsilon)R1beta gene polymorphisms in susceptibility to atopy in Korean children with asthma. Eur J Pediatr. 2009; 168:1483–1490. PMID:
19288130.
54. Chae SC, Park BL, Park CS, Ryu HJ, Yang YS, Lee SO, Choi YH, Kim EM, Uh ST, Kim YH, Kim KK, Oh B, Chung HT, Kimm K, Shin HD. Putative association of RUNX1 polymorphisms with IgE levels in a Korean population. Exp Mol Med. 2006; 38:583–588. PMID:
17079875.

55. Park JH, Chang HS, Park CS, Jang AS, Park BL, Rhim TY, Uh ST, Kim YH, Chung IY, Shin HD. Association analysis of CD40 polymorphisms with asthma and the level of serum total IgE. Am J Respir Crit Care Med. 2007; 175:775–782. PMID:
17255560.

56. Kim YK, Park HW, Yang JS, Oh SY, Chang YS, Shin ES, Lee JE, Kim S, Gho YS, Cho SH, Min KU, Kim YY. Association and functional relevance of E237G, a polymorphism of the high-affinity immunoglobulin E-receptor beta chain gene, to airway hyper-responsiveness. Clin Exp Allergy. 2007; 37:592–598. PMID:
17430357.
57. Kim HB, Kang MJ, Lee SY, Jin HS, Kim JH, Kim BS, Jang SO, Lee YC, Sohn MH, Kim KE, Hong SJ. Combined effect of tumour necrosis factor-alpha and interleukin-13 polymorphisms on bronchial hyperresponsiveness in Korean children with asthma. Clin Exp Allergy. 2008; 38:774–780. PMID:
18341619.
58. Sohn MH, Kim SH, Kim KW, Jee HM, Park HS, Kim KE. RANTES gene promoter polymorphisms are associated with bronchial hyperresponsiveness in Korean children with asthma. Lung. 2008; 186:37–43. PMID:
17990036.

59. Hong SJ, Kim HB, Kang MJ, Lee SY, Kim JH, Kim BS, Jang SO, Shin HD, Park CS. TNF-alpha (-308 G/A) and CD14 (-159T/C) polymorphisms in the bronchial responsiveness of Korean children with asthma. J Allergy Clin Immunol. 2007; 119:398–404. PMID:
17196641.
60. Park HW, Shin ES, Lee JE, Kwon HS, Chun E, Kim SS, Chang YS, Kim YK, Min KU, Kim YY, Cho SH. Multilocus analysis of atopy in Korean children using multifactor-dimensionality reduction. Thorax. 2007; 62:265–269. PMID:
17121871.
61. Lee JH, Moore JH, Park SW, Jang AS, Uh ST, Kim YH, Park CS, Park BL, Shin HD. Genetic interactions model among Eotaxin gene polymorphisms in asthma. J Hum Genet. 2008; 53:867–875. PMID:
18712274.

62. Cho SH, Oh SY, Bahn JW, Choi JY, Chang YS, Kim YK, Min KU, Kim YY. Association between bronchodilating response to short-acting beta-agonist and non-synonymous single-nucleotide polymorphisms of beta-adrenoceptor gene. Clin Exp Allergy. 2005; 35:1162–1167. PMID:
16164442.
63. Park HW, Yang MS, Park CS, Kim TB, Moon HB, Min KU, Kim YY, Cho SH. Additive role of tiotropium in severe asthmatics and Arg16Gly in ADRB2 as a potential marker to predict response. Allergy. 2009; 64:778–783. PMID:
19183167.
64. Kim JH, Lee SY, Kim HB, Jin HS, Yu JH, Kim BJ, Kim BS, Kang MJ, Jang SO, Hong SJ. TBXA2R gene polymorphism and responsiveness to leukotriene receptor antagonist in children with asthma. Clin Exp Allergy. 2008; 38:51–59. PMID:
18031559.

65. Palikhe NS, Kim SH, Park HS. What do we know about the genetics of aspirin intolerance? J Clin Pharm Ther. 2008; 33:465–472. PMID:
18834360.

66. Kim SH, Lee JE, Jee YK, Kim YK, Park HS, Min KU, Park HW. Allelic variants of CD40 and CD40L genes interact to promote antibiotic-induced cutaneous allergic reactions. Clin Exp Allergy. 2009; 39:1852–1856. PMID:
19735272.

67. Kim TB, Park CS, Bae YJ, Cho YS, Moon HB. Factors associated with severity and exacerbation of asthma: a baseline analysis of the cohort for reality and evolution of adult asthma in Korea (COREA). Ann Allergy Asthma Immunol. 2009; 103:311–317. PMID:
19852195.

68. Cho SH, Kim YK, Chang YS, Kim SS, Min KU, Kim YY. Asthma insights and reality in Korea. Korean J Med. 2006; 70:69–77. Korean.
69. The Korean Academy of Asthma, Allergy and Clinical Immunology. National guideline for the management of asthma. J Asthma Allergy Clin Immunol. 1998; 18:339–384. Korean.
70. Choi BW, Yoo KH, Jeong JW, Yoon HJ, Kim SH, Park YM, Kim WK, Oh JW, Rha YH, Pyun BY, Chang SI, Moon HB, Kim YY, Cho SH. Easy diagnosis of asthma: computer-assisted, symptom-based diagnosis. J Korean Med Sci. 2007; 22:832–838. PMID:
17982231.

71. Cho SH, Jeong JW, Park HW, Pyun BY, Chang SI, Moon HB, Kim YY, Choi BW. Effectiveness of computer-assisted asthma management program on physician adherence to guideline. J Asthma. 2010; Forthcoming.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download