Abstract
Anaphylaxis is a potentially life-threatening systemic allergic reaction, often with an explosive onset; the symptoms range from mild flushing to upper respiratory obstruction, with or without vascular collapse. Foods are common offending allergens and remain the leading cause of outpatient anaphylaxis in most surveys. Yacon (Smallanthus sonchifolius) is a plant native to the Andes region, where its root is cultivated and consumed mainly as food. Unlike most edible roots, yacon contains large amounts of ructooligosaccharides. Traditionally, yacon tubers have been used as a source of natural sweetener and syrup for people suffering from various disorders. We report the case of a 55-year-old woman who developed syncope and generalized urticaria after ingesting yacon roots. The patient had positive skin prick and intradermal tests to yacon extract. An open food challenge test was performed to confirm food anaphylaxis and was positive 10 minutes after the consumption of yacon roots. To our knowledge, this is the first reported case of anaphylaxis after the ingestion of yacon roots.
Yacon (Smallanthus sonchifolius) (Fig. 1), a native to the Andes Mountains in South America, is a perennial bulbous plant that belongs to the family Compositae. It was introduced to Korea in 1985.1 Yacon roots look like sweet potatoes, taste like pears, and are very juicy. Unlike other bulbous plants, yacon roots contain large amounts of fructooligosaccharides (40~70%) and thus are used as a natural sweetener. Yacon roots are also reported to have beneficial effects in obesity and insulin resistance in humans.2 Chen et al.3 demonstrated that yacon roots exert a favorable effect on bowel function and nutritional status in constipated elderly men. In addition, yacon roots reduced blood glucose and cholesterol levels in patients with metabolic syndrome and diabetes mellitus.4,5 As the popularity of health foods has increased, yacon roots have become known to the general population. Owing to their specific pharmacological action and peculiar taste, yacon roots have been introduced as a functional food. No anaphylactic reaction to yacon roots has ever been reported worldwide. Here, we report a 55-year-old woman who developed anaphylaxis after ingesting yacon roots and was definitively diagnosed with anaphylaxis to yacon roots based on skin and provocation testing with yacon extract.
A 55-year-old woman developed generalized urticaria, itching, and a subsequent coma within 5 minutes of ingesting yacon roots. At the time, she was in a steam bath. She was treated at a regional hospital, and her systolic blood pressure was 60 mmHg. She was then referred to our clinic to determine the cause of her problem.
She had a 20-year history of hypothyroidism and had been taking amlodipine (5 mg) for hypertension for 3 years. There was no history of bronchial asthma, allergic rhinitis, or atopic dermatitis, although she frequently developed pruritus when preparing Codonopsis lanceolata. She did not smoke or consume alcohol. She reported that her one son developed urticaria and angioedema when he ingested shrimp, apple, or pear.
On physical examination, she appeared acutely ill, but her consciousness was clear. Her blood pressure was 100/60 mmHg; respiratory rate, 20/minute; pulse, 81/minute; and body temperature, 36℃. Her conjunctivae were not anemic, and no jaundice was observed in her sclera. On auscultation, no rales or stridor were heard. There were no abnormal findings in the abdomen or extremities.
Routine blood tests revealed a white blood cell count of 5,350/µL, 182/µL eosinophils (3.4% of all white blood cells), and an erythrocyte sedimentation rate (ESR) of 2 mm/hour. Thyroid function tests showed TSH at 5.99 mIU/L (normal range, 29-4.2 mIU/L), free T4 at 0.86 ng/dL (normal, 0.93-1.70 ng/dL), total T3 at 96.14 ng/dL (normal, 80-200 ng/dL), and anti-TPO antibody at 278.3 IU/mL (normal, 0-314 IU/mL). No abnormalities were observed by biochemical or serologic testing, urinalysis, stool examination, electrocardiography, and simple chest X-rays.
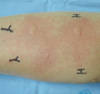
She refused skin prick tests with common inhalant allergens, although skin prick and intradermal tests were performed using juice extracted from cut raw yacon root. The skin prick test showed a 3.5×4.0/+ reaction to histamine (H), -/- reaction to normal saline, 4.5×7.0/+ reaction to yacon extract (allergen A), and a 3+ A/H ratio (positive reaction) (Fig. 2). An oral food provocation test was performed using 1-g pieces of yacon root. The patient complained of tingling, pain, and numbness in the oral cavity and around the lips at about 5 minutes after ingesting 2 g of the root. Headache, chest tightness, dizziness, and pruritus/urticaria extending to the neck and face developed about 10 minutes after ingesting the root. At that time, her blood pressure was 85/50 mmHg and her pulse was 115/minute. The oral provocation test was stopped because the patient complained of chest tightness and dizziness. The patient was given epinephrine (1:1,000, 3 mL, intramuscularly), dexamethasone (5 mg, intravenously), and fluids. Her symptoms improved immediately and her blood pressure normalized. She was definitively diagnosed with anaphylaxis to yacon root based on the oral provocation test results. She was instructed not to ingest yacon roots and to carry a portable epinephrine kit for use in an emergency. Levothyroxine (50 µg) was prescribed for her hypothyroidism.
With the increased popularity of the concept of well-being and increased interest in personal health, a variety of health foods have been developed. Yacon has been consumed as a health food, despite insufficient evidence of its beneficial effects on diabetes mellitus, renal disease, and gastrointestinal disease.2,3 Yacon roots have a diameter similar to that of dahlia, another plant in the family Compositae. Fructooligosaccharides, which are present in yacon roots, are reported to have a beneficial effect on the normal intestinal flora and to prevent constipation.6-8 In addition, the polyphenol component of yacon roots or leaf extracts has antioxidative properties, anticancer activity, and a prophylactic effect in arteriosclerosis.9,10
Yacon is not widely known in Korea. Although the effects of yacon roots have been reported to some extent, few studies have examined adverse reactions, especially anaphylaxis, induced by yacon roots. Seminario et al.11 reported the development of mild diarrhea after ingestion of large amounts of yacon roots. Genta et al.12 reported the first study on adverse reactions and the toxicity of yacon roots. In their rat experiment, the rats were divided into a control group that was fed a basic diet and two experimental groups that were fed a basic diet mixed with low or high concentrations of yacon powder for 4 months. They found no significant differences in adverse reactions, toxicity, or nutritional disturbances among the three groups, although the appendix was enlarged in a few rats in the high-concentration yacon group. Valentova et al.13 reported adverse reactions to yacon roots in 101 patients with metabolic syndrome in a 90-day randomized placebo-controlled study using maca (a high-nutrition diet used in the Andes Mountains) and yacon in combination with silymarin (a therapeutic agent for hepatitis), although the frequency of adverse reactions did not differ significantly between the control and experimental groups.
Ours is the first case of anaphylaxis to yacon reported worldwide. Anaphylaxis is a systemic allergic disease that occurs immediately after exposure to an offending allergen; it manifests as various symptoms such as mild angioedema, airway obstruction, hypotension, or cardiovascular shock.14 Its diagnosis can be established after a comprehensive assessment of the history of exposure to offending allergens, the time interval between the exposure and symptom onset, and clinical features suggestive of anaphylaxis, including pruritus, angioedema, urticaria, dizziness, coma, dyspnea, and hypotension. Our patient was readily diagnosed with anaphylaxis to yacon (potentially the primary offending allergen) because she developed urticaria, pruritus, dizziness, coma, and hypotension about 10 minutes after ingesting yacon roots. Generally, standard laboratory tests are not useful for the diagnosis of anaphylactic reactions. The serum tryptase level is more useful than the serum histamine level for diagnosing anaphylactic reactions, although the serum tryptase level is not always increased in patients with food-induced anaphylaxis.15 A plausible explanation for this is that basophils are predominantly involved in the reaction, rather than mast cells. An assessment of the factors activating basophils and mast cells, including beta-tryptase, mast cell carboxypeptidase A3, chymase, and platelet-activating factor, will help to diagnose anaphylactic reactions.16,17
IgE-mediated anaphylaxis can be diagnosed with serum tests using specific IgEs against food allergens and with skin tests using suspected food allergens. In our patient, the presence of specific IgE against yacon roots was not confirmed. Although we could not detect serum specific IgE to yacon roots, we speculate that the pathogenic mechanism was IgE-mediated, based on strong positive results to the skin test and the immediate onset of urticaria and anaphylaxis. Food allergy can be diagnosed definitively using a double-blind placebo-controlled oral food provocation test, which is accepted as the gold standard test for food allergy because it excludes physician and patient bias.18,19 However, a double-blind placebo-controlled provocation test has the disadvantages of difficulty in selecting an appropriate placebo and possible changes in food allergens during the preparation process. Food allergy can be diagnosed using an open oral food provocation test alone, when the test results are interpreted using objective criteria. In our case, an open oral food provocation test was used to diagnose the anaphylactic reaction to yacon roots. The provocation test was stopped because the clinical symptoms and signs of anaphylaxis occurred about 10 minutes after the ingestion of yacon roots. The patient was given epinephrine (1:1,000; 3 mL; intramuscularly), dexamethasone (5 mg, intravenously), and fluids; her symptoms improved immediately and her blood pressure normalized. Based on these findings, she was definitively diagnosed with anaphylaxis to yacon roots. The patient was instructed to not ingest yacon roots and to use a portable epinephrine kit in emergency situations.
We report a case of anaphylaxis caused by yacon roots in which an IgE-mediated mechanism was postulated as the pathogenic mechanism.
Figures and Tables
References
1. Kin SJ, Jung JH. Study on development of South American roots. Report of horticultural laboratory (department of vegetable). 1986. 99–101.
2. Genta S, Cabrera W, Habib N, Pons J, Carillo IM, Grau A, Sánchez S. Yacon syrup: beneficial effects on obesity and insulin resistance in humans. Clin Nutr. 2009. 28:182–187.
3. Chen H-L, Lu Y-H, Lin J Jr, Ko L-Y. Effects of fructooligosaccharide on bowel function and indicators of nutritional status in constipated elderly men. Nutr Res. 2000. 20:1725–1733.
4. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA. 2001. 285:2486–2497.
5. Valentová K, Moncion A, de Waziers I, Ulrichová J. The effect of Smallanthus sonchifolius leaf extracts on rat hepatic metabolism. Cell Biol Toxicol. 2004. 20:109–120.
6. Pedreschi R, Campos D, Noratto G, Chirinos R, Cisneros-Zevallos L. Andean yacon root (Smallanthus sonchifolius Poepp. Endl) fructooligosaccharides as a potential novel source of prebiotics. J Agric Food Chem. 2003. 51:5278–5284.
7. Geyer M, Manrique I, Degen L, Beglinger C. Effect of yacon (Smallanthus sonchifolius) on colonic transit time in healthy volunteers. Digestion. 2008. 78:30–33.
8. Lachman J, Fernández EC, Orsák M. Yacon [Smallanthus sonchifolia (Poepp. et Endl.) H. Robinson] chemical composition and use-a review. Plant Soil Environ. 2003. 49:283–290.
9. Valentová K, Ulrichová J. Smallanthus sonchifolius and Lepidium meyenii - prospective Andean crops for the prevention of chronic diseases. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2003. 147:119–130.
10. Valentová K, Sersen F, Ulrichová J. Radical scavenging and anti-lipoperoxidative activities of Smallanthus sonchifolius leaf extracts. J Agric Food Chem. 2005. 53:5577–5582.
11. Seminario J, Valderrama M, Manrique I. El yacón: fundamentos para el aprovechamiento de un recurso promisorio. 2003. Lima, Perú: Centro Internacional de la Papa (CIP), Universidad nacional de Cajamarca, Agencia Suiza Para el Desarrollo y la Cooperación (COSUDE);60.
12. Genta SB, Cabrera WM, Grau A, Sánchez SS. Subchronic 4-month oral toxicity study of dried Smallanthus sonchifolius (yacon) roots as a diet supplement in rats. Food Chem Toxicol. 2005. 43:1657–1665.
13. Valentová K, Stejskal D, Bartek J, Dvorácková S, Kren V, Ulrichová J, Simánek V. Maca (Lepidium meyenii) and yacon (Smallanthus sonchifolius) in combination with silymarin as food supplements: in vivo safety assessment. Food Chem Toxicol. 2008. 46:1006–1013.
14. Adkinson NF Jr, Bochner BS, Busse WW, Holgate ST, Lemanske RF Jr, Simons FE. Lieberman PL, editor. Anaphylaxis. Middleton's Allergy Principles & Practice. 2009. 7th ed. Philadelphia: Elsevier Inc.;1027–1049.
15. Sampson HA, Mendelson L, Rosen JP. Fatal and near-fatal anaphylactic reactions to food in children and adolescents. N Engl J Med. 1992. 327:380–384.
16. Vadas P, Gold M, Perelman B, Liss GM, Lack G, Blyth T, Simons FE, Simons KJ, Cass D, Yeung J. Platelet-activating factor, PAF acetylhydrolase, and severe anaphylaxis. N Engl J Med. 2008. 358:28–35.
17. Simons FE, Frew AJ, Ansotegui IJ, Bochner BS, Golden DB, Finkelman FD, Leung DY, Lotvall J, Marone G, Metcalfe DD, Müller U, Rosenwasser LJ, Sampson HA, Schwartz LB, van Hage M, Walls AF. Risk assessment in anaphylaxis: current and future approaches. J Allergy Clin Immunol. 2007. 120:1 Suppl. S2–S24.
18. Wang J, Sampson HA. Food anaphylaxis. Clin Exp Allergy. 2007. 37:651–660.
19. Sicherer SH, Sampson HA. 9. Food allergy. J Allergy Clin Immunol. 2006. 117:2 Suppl Mini-Primer. S470–S475.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download