Abstract
Purpose
Rhinitis and asthma usually occur together. There are increasing evidences that allergic rhinitis (AR) may influence the clinical course of asthma. The aim of this study is to evaluate clinical parameters and therapeutic response in patients with between asthma and asthma with AR.
Methods
Four-hundred eighty-five patients with asthma and 428 asthmatics with AR, who had lesser than 50 years old and smoked less than 10 pack-years were recruited. We compared FEV1 and FEV1/FVC following bronchodilator, atopy, IgE, emphysema on HRCT, and aspirin intolerance between two groups. Also we compared physiologic fixed airway obstruction defined using FEV1/FVC and FEV1 less than 75% following anti-asthmatic
drug for 1 year.
Results
46.8% (428/913) asthmatics suffered from AR. There were no differences of total IgE, body mass index, PC20, sputum eosinophils and emphysema on HRCT between two groups. The age in asthmatics was higher than that in those with AR. FEV1/FVC was lower in asthmatics than in those with AR. The prevalence of atopy was higher in asthmatics with AR than in asthmatics. Aspirin intolerance was higher in asthmatics with AR than in asthmatics (42/218 vs 13/109, P=0.001). Fixed airway obstruction were more observed in asthmatics than in those with AR (39/319 vs 28/355, P=0.001) after anti-asthmatic drug for 1 year.
Close association exists between allergic rhinitis (AR) and asthma. There are increasing evidences that AR may influence the clinical course of asthma adults.1-6
Putative mechanisms linking rhinitis to asthma are explained by direct and indirect effect.7 The direct effects are naso-bronchial reflex, postnasal drip of inflammatory cells and/or mediators from the nose into the lower airways, and absorption of inflammatory cells and/or mediators from the nose into the systemic circulation and ultimately the lung. The indirect effects are nasal obstruction causing reduction in filtration, humidification, and warming function of the nose.8
Rhinitis patients showed a lower degree of bronchial hyperresponsiveness (BHR) to allergen than asthmatics, but responded to allergen inhalation with changes in airway inflammation and in maximal response plateau very similar to asthmatics.8 The differences between asthma and AR in symptoms depend on a quantitatively different response to environmental allergen inhalation.8
Eosinophilic inflammation may be present in subjects with AR and BHR even when there are no symptoms of asthma.9
There is a little report about comparative data of clinical and therapeutic response between asthmatics and asthmatics with AR. The aim of this study is to evaluate clinical parameters and therapeutic response to asthma between asthmatics and asthmatics with AR.
Subjects were recruited from the Genome Research Center for Allergy and Respiratory Diseases at Soonchunhyang University, Bucheon, Korea, and Chunan Hospital, Chunan, Korea. All patients were diagnosed by a physician and met the definition of asthma set forth in the Global Initiative for Asthma (GINA) guidelines.10 All patients had a history of dyspnea and wheezing during the previous 12 months, plus one of the following: 1) >15% increase in FEV1 or >12% increase plus 200 mL following inhalation of a short-acting bronchodilator; 2) <10 mg/mL PC20 methacholine; and 3) >20% increase in FEV1 following 2 weeks of treatment with inhaled steroids and long-acting bronchodilators.10
Symptoms of allergic rhinitis were assessed by questionnaires on Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update.11 The symptoms of AR is on responses to questionnaires on 'do you have any nasal allergies including anterior or posterior rhinorrhoea, sneezing, nasal blockage and/or itching of the nose?' and 'do you have your symptoms occur during two or more consecutive days for more than 1 h on most days?' and 'do you have symptoms during season or perennial?' and 'do your symptoms impair of daily activities and school or work?'. All patients were diagnosed by a physician and met the definition of AR set forth in the Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update.11
The exclusion criteria included greater than 10 pack-years smoking, less than 20 or more than 50 years old, parenchymal lung disease apparent on chest radiography, diffusing capacity less than 80%, severe uncontrolled diabetes mellitus, and renal, hepatic, or cardiac failure. This study was performed with the approval of the Ethics Committee of the University Hospital, and informed written consent was obtained from all of the study subjects.
Study patients received long-term control medication consisting of inhaled glucocorticoids or inhaled glucocorticoids plus long-acting inhaled β2 agonists or sustained-release theophylline or a leukotriene modifier or oral glucocorticoids according to GINA guidelines,10 and rapid relief medication consisting of two puffs of inhaled salmeterol (100 µg per dose), and two puffs of inhaled ipratropium bromide (20 µg per dose) on an as-needed basis.
Fixed airway obstruction as a functional marker of remodeling was defined as forced expiratory volume in one second/forced vital capacity (FEV1/FVC) and a predicted FEV1 of less than 75% following bronchodilator.12 The emphysema on HRCT, near-fatal asthma attacks, asthma duration, atopy, sex, age, and body mass index (BMI) were compared in asthmatics and asthmatics with AR following anti-asthmatic drug for 1 year.
Baseline FVC and FEV1 measurements were obtained in the absence of recent bronchodilator use (within 8 h) and selected according to the American Thoracic Society criteria.13 Basal and post-bronchodilator FEV1, FVC, and forced expiratory flow between 25 and 75% FVC (FEF25-75%) were measured between 1:00 and 4:00 pm. AHR was measured using the methacholine challenge and was expressed as the provocation concentration that caused a fall in FEV1 of 20% (PC20), in non-cumulative units.14
Sputum15 was induced using isotonic saline that contained a short-acting bronchodilator, as described by Norzila et al.16 Samples were treated within 2 h of collection using the method of Pizzichini et al.17 with a minor modification.
Briefly, all of the visible portions with greater solidity were carefully selected and placed in a pre-weighed Eppendorf tube. The samples were treated by adding eight volumes of 0.05% dithiothreitol (Sputolysin; Calbiochem, San Diego, CA, USA) in Dulbecco's phosphate-buffered saline (D-PBS). One volume of protease inhibitor (0.1 M EDTA and 2 mg/mL phenylmethylsulfonylfluoride) was added to 100 volumes of homogenized sputum, and the total cell count was determined with a hemocytometer. The homogenized sputum was spun in a cytocentrifuge, and 500 cells were read on each sputum slide stained with Diff-quick solution (American Scientific Products, Chicago, IL, USA).
Allergy skin prick tests were performed using using 24 commercial
inhalant allergens (Bencard, UK), which included Dermatophagoides
farinae and D. pteronyssinus, cat fur, dog fur, cockroaches, and grasses, trees, and ragweed pollens and histamine (1 mg/mL). None of the subjects had received antihistamines orally in the 3 days preceding the study. All of the tests included positive (1 mg/mL histamine) and negative (diluent) controls. After 15 min, the mean diameter of the wheals formed by the allergens was compared with that formed by histamine. When the former was the same as or larger than the latter (A/H ratio ≥1.0), the reaction was deemed positive. Atopy was determined by the presence of an immediate skin reaction to one or more aeroallergens, as described previously.18
Body mass index (BMI) as an obesity marker was calculated as weight/height2 (kg/m2).
The oral provocation test was performed with increasing doses of aspirin (10-450 mg Astrix; Mayne Pharma Ltd., Melbourne, Australia) using a slightly modified method from those described previously.19,20 Provocations always started between 08:00 and 09:00 AM. Antihistamine and short-acting 2-agonists were withdrawn for 24 h. Changes in FEV1 were followed for 5 h after the last aspirin challenge dose. The maximum fall in FEV1 during the follow-up period was used as the value for the decline of FEV1 by aspirin provocation. Aspirin-induced bronchospasm as reflected by rate of FEV1 decline was calculated as the pre-challenge FEV1 minus the post-challenge FEV1 divided by the pre-challenge FEV1. Subjects were labeled as positive responders if they showed the rate of FEV1 decline of more than 15% or skin manifestations. Subjects exhibiting rate of FEV1 decline below 15% and absence of nasal or skin symptoms were regarded as negative responders (ATA).
Thin-slice CT scanning and radiological evaluation. Patients underwent volumetric thin section CT scanning of the chest using a Somatom 4 scanner (Siemens Medical Systems, Forchheim, Germany) as previously described.21-23
Patients were scanned caudocranially in one breath hold; 1 mm collimation was used at a table feed of 6 mm/0.75 s scanner rotation (8 mm/s) at 120 kV and 140 mA. For the expiratory thin section CT scan, all subjects were instructed to take a deep breath, exhale all the way, and hold their breath. Scanning was performed from the lung bases toward the apices. The volumetric axial images with 1 mm thickness and the 10 mm intervals were reconstructed with a high spatial frequency algorithm on both end inspiration and end expiration scanning. All scans were obtained at suspended end inspiratory volume because artifacts have been reported in scans obtained at functional residual capacity.24 The images were viewed at two window levels of 2,450 HU for accurate measurement of bronchial diameters and 2,700 HU for analysis of other HRCT features. All images were displayed at the lung window setting using a PACS (picture archiving and communication system) workstation (Starpacs, Infinitt Technology). The thin section CT scans were evaluated for the presence and/or extent of the emphysema. These findings were defined according to the glossary of terms recommended by the Fleischner Society.24
Variables differences were compared at the period of asthma and AR diagnosis and the physiologic fixed airway obstruction following anti-asthmatic drug for 1 year were compared. Group differences were compared using two-sample t-tests, Wilcoxon rank-sum tests, and the Pearson χ2 test for normally distributed, skewed, and categorical data, respectively. A P value less than 0.05 was considered statistically significant.
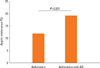
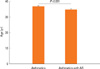
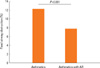
The 46.8% (428/913) asthmatics suffered from AR (Table 1). There were no differences of total IgE (263.1±25.5 IU vs 318.3±20.4 IU), body mass index (23.1±0.2 kg/m2 vs 23.1±0.3), PC20 (6.06±0.44 mg/mL vs 6.31±0.47 mg/mL), sputum eosinophils (3.20±0.67% vs 3.43±0.62%), sputum neutrophils (26.0±2.11% vs 24.9±2.06%), and emphysema on HRCT (1/36 vs 2/40) in between asthmatics and those with AR. Asthma duration was not different between asthmatics and those with rhinitis. The age in asthmatics was higher than that in asthmatics with AR (36.7±0.4 vs 34.8±0.45, P=0.001, Fig. 1). Although there were no differences of post bronchodilator FEV1 % predicted (86.4±1.15% vs 86.7 ±1.00%) and post bronchodilator FVC % predicted (86.3±0.97% vs 85.8±0.88%) and post bronchodilator FEF (75.8±1.67% vs 79.5±1.60%) in two groups, post bronchodilator FEV1/FVC (79.4±0.59% vs 81.3±0.53%, P=0.018, Fig. 2) was lower in asthmatics than in those with AR. The prevalence of atopy [296/428 (69.1%) vs 232/485 (47.8%), P=0.001] was higher in asthmatics with AR than in asthmatics. The prevalence of aspirin intolerance in tested patients was 10.09% (55/545). Aspirin intolerance was higher in asthmatics with AR than in asthmatics [42/218 (19.2%) vs 13/109 (11.9%), P=0.001, Fig. 3]. Fixed airway obstruction defined as a functional marker of remodeling was defined as FEV1/FVC and a predicted FEV1 of less than 75% following bronchodilator were more observed in asthmatics than in those with AR [39/319 (12.2%) vs 28/355 (7.8%), P=0.001, Fig. 4] after treatment with inhaled steroid and other anti-asthma medications for no less than one year.
Asthmatics with AR had more atopy prevalence and aspirin intolerance than those in asthmatics, and asthmatics had poor response to anti-inflammatory drugs than in asthmatics with AR, indicating that asthmatics have more fixed airway obstruction than combined AR in asthmatics and concurrent rhinitis in asthmatics may be a favorable factor in terms of treatment.
There is a link between AR and asthma. Asthma and AR can be associated both with an IgE-mediated allergic reaction and an inflammatory pattern. 28-50% of asthmatic patients had AR compared to 10-20% in the general population.6 AR is associated to asthma and constitutes an independent risk factor for its occurrence.1 Our data showing 46.8% prevalence of AR in the asthmatics is in good agreement with the previous reports. And asthmatics with AR had more atopy prevalence than asthmatics, indicating that combined AR in asthmatics be positive skin test results to common aeroallergens.
Many patients with AR who have no perceived asthma symptoms have BHR to natural stimuli such as exercise or to bronchial challenge with chemical stimuli such as histamine and methacholine, especially during AR exacerbation.6 Simons25
suggested that the new term "allergic rhinobronchitis" accurately describe chronic allergic inflammation throughout the airways of patients with concurrent AR and asthma and recommended the key to management of both disorders lies in addressing the common immunopathologic mechanisms and in preventing and relieving chronic allergic inflammation, not only with appropriate pharmacologic treatment but also by recommending allergen avoidance and in selected patients, specific immunotherapy. When it comes to looking at patients with asthma we should considerate combined asthma and rhinitis to treat both asthma and AR.
The underlying pathologic processes are similar in the upper and lower airways.3 Immune effector cells responsible for allergic reactions in both the lung and nose include, most prominently, mast cell, T lymphocytes, and eosinophils.26-28 Eosinophils
are characteristic for acute and chronic inflammatory changes observed in asthma and AR and have also been implicated in many aspects of tissue damage that occurs at sites of chronic inflammation. In this study there are no differences of sputum eosinophils between asthmatics and asthmatics with AR. Sputum eosinophils did not make a differentiation between asthmatics and asthmatics with AR. Further studies will be needed to define the role of inflammatory cells between upper and lower airways.
AR patients who had hyperresponsive to methacholine were at significantly greater risk of developing asthma than those with normal bronchial challenge.29 Upper airway inflammatory processes occurring totally or primarily in the upper airway may participate in the pathogenesis of BHR and asthma.29 Rhinitis subjects with BHR develop asthma more frequently than those without.6,29 In this study there are no differences of PC20 between BA and BA with AR. Further studies would be needed to determine the time point change of AR to BA.
Airway remodeling plays an important role in the pathophysiology of asthma phenotypes, such as BHR and airway inflammation, in patients with asthma.30-34 Airway remodeling is linked to AHR via diverse triggers and a steeper trajectory of the long-term decrease in lung function in asthmatic patients.35
Common mucosal inflammatory responses occur in combined AR and asthma syndrome. The current best practice is to treat asthma conventionally with inhaled corticosteroid with or without β2 agonist and to add intranasal corticosteroid to improve specific rhinitis symptoms.36,37 In this study fixed airway obstruction defined as a functional marker of remodeling was defined as FEV1/FVC and a predicted FEV1 of less than 75% following bronchodilator12 were more observed in asthmatics than in asthmatics with AR after anti-inflammatory drug for 1 year.
The authors12,37,38 reported that non atopy, asthma duration, emphysema on high-resolution computed tomography, sputum eosinophils, age, and BMI before antiasthma treatment are important factors related to airway remodeling in patients with asthma and the FEV1 percent predicted and the blood and sputum eosinophil levels prior to GC inhalation are associated with the responsiveness to inhaled GCs in patients with moderateto severe asthma
Our study suggest that non-atopy and emphysema on HRCT, and age may be important discriminators of asthmatics with AR and asthmatics and asthmatics with concurrent AR may have suffered from diseases for longer duration in agreement to previous studies.12,37,38 Those results imply that just asthmatics have more airway remodeling and early diagnosis and early treatment is necessary for preventing airway remodeling.
Aspirin and non-steroidal anti-inflammatory drugs (NSAID) specifically inhibit cyclo-oxygenase (prostaglandin-endoperoxide synthase), leading to a reduction in prostaglandin E2 and overproduction of cysteinyl leukotrienes.39 Aspirin-intolerant asthma (AIA), the development of bronchoconstriction in asthmatic individuals following the ingestion of aspirin or other NSAID, affects 5-10% of asthmatic adults.40,41 In this study, we compared aspirin provocation tests between BA and BA with AR. Patients with BA with AR had more aspirin tolerance, suggesting that combined AR in asthmatics have more aspirin intolerance and especially those patients should treated cautiously by aspirin. Our results in the contrary of previous reports40,41 that AIA is more prevalent in non-allergic rhinosinusitis may be possible due to only including patients done with aspirin provocation test.
In conclusion asthmatics with AR had more atopy and aspirin intolerance than those in asthmatics, and asthmatics had poor response to anti-inflammatory drugs than in asthmatics with AR, indicating that combined AR in asthmatics may be a favorable factor in terms of treatment and we should treat concurrently both asthma and AR.
Figures and Tables
Fig. 2
Lung function following bronchodilator between asthmatics and those with allergic rhinitis. A: asthmatics, B: asthmatics with allergic rhinitis. FEV1, forced expiratory volume in one second; FVC, forced vital capacity.
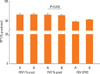
Fig. 3
Aspirin intolerance between asthmatics and those with AR. Aspirin intolerance defined as showing symptoms with a fall in FEV1 greater than 15% following aspirin provocation test in patients.
ACKNOWLEDGMENTS
The study was supported by the Korea Health 21 R&D Project, Ministry for Health, Welfare and Family Affairs, Republic of Korea (A090548 and A040153).
References
1. Corren J. The connection between allergic rhinitis and bronchial asthma. Curr Opin Pulm Med. 2007. 13:13–18.
2. Bachert C, Patou J, Van Cauwenberge P. The role of sinus disease in asthma. Curr Opin Allergy Clin Immunol. 2006. 6:29–36.
3. Ciprandi G, Cirillo I. The lower airway pathology of rhinitis. J Allergy Clin Immunol. 2006. 118:1105–1109.
4. Townley RG, Ryo UY, Kolotkin B, Kang B. Bronchial sensitivity to methacholine in current and former asthmatic and allergic rhinitis patients and control subjects. J Allergy Clin Immunol. 1975. 56:429–442.
5. Madonini E, Briatico-Vangosa G, Pappacoda A, Maccagni G, Cardani A, Saporitu F. Seasonal increase of bronchial reactivity in allergic asthma. J Allergy Clin Immunol. 1987. 79:358–363.
6. Ramsdale EH, Morris MM, Roberts RS, Hargreave FE. Asymptomatic bronchial hyperresponsiveness in rhinitis. J Allergy Clin Immunol. 1985. 75:573–577.
7. Corren J. The impact of allergic rhinitis on bronchial asthma. J Allergy Clin Immunol. 1998. 101:S352–S356.
8. Alvarez MJ, Olaguibel JM, Garcia BE, Tabar AI, Urbiola E. Comparison of allergen-induced changes in bronchial hyperresponsiveness and airway inflammation between mildly allergic asthma patients and allergic rhinitis patients. Allergy. 2000. 55:531–539.
9. Gutierrez V, Prieto L, Torres V, Morales C, Gonzalez E. Peak flow variability and sputum eosinophilia in allergic rhinitis. Ann Allergy Asthma Immunol. 1998. 81:143–150.
10. Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention. NHLBI/WHO workshop report. Bethesda (MD): National Institutes of Health National Heart, Lung, and blood Institute; 2002. NIH publication no. 02-3659. J Allergy Clin Immunol. 2002. 110(5 Suppl):S141–S219.
11. Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, Togias A, Zuberbier T, Baena-Cagnani CE, Canonica GW, van Weel C, Agache I, Aït-Khaled N, Bachert C, Blaiss MS, Bonini S, Boulet LP, Bousquet PJ, Camargos P, Carlsen KH, Chen Y, Custovic A, Dahl R, Demoly P, Douagui H, Durham SR, van Wijk RG, Kalayci O, Kaliner MA, Kim YY, Kowalski ML, Kuna P, Le LT, Lemiere C, Li J, Lockey RF, Mavale-Manuel S, Meltzer EO, Mohammad Y, Mullol J, Naclerio R, O'Hehir RE, Ohta K, Ouedraogo S, Palkonen S, Papadopoulos N, Passalacqua G, Pawankar R, Popov TA, Rabe KF, Rosado-Pinto J, Scadding GK, Simons FE, Toskala E, Valovirta E, van Cauwenberge P, Wang DY, Wickman M, Yawn BP, Yorgancioglu A, Yusuf OM, Zar H, Annesi-Maesano I, Bateman ED, Ben Kheder A, Boakye DA, Bouchard J, Burney P, Busse WW, Chan-Yeung M, Chavannes NH, Chuchalin A, Dolen WK, Emuzyte R, Grouse L, Humbert M, Jackson C, Johnston SL, Keith PK, Kemp JP, Klossek JM, Larenas-Linnemann D, Lipworth B, Malo JL, Marshall GD, Naspitz C, Nekam K, Niggemann B, Nizankowska-Mogilnicka E, Okamoto Y, Orru MP, Potter P, Price D, Stoloff SW, Vandenplas O, Viegi G, Williams D; World Health Organization; GA(2)LEN; AllerGen. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen). Allergy. 2008. 63:8–160.
12. Jang AS, Lee JH, Park SW, Park JS, Kim DJ, Park CS. Risk factors related to fixed airway obstruction in patients with asthma after antiasthma treatment. Ann Allergy Asthma Immunol. 2007. 99:408–412.
13. Standards for. COPD) and asthma. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, November 1986. Am Rev Respir Dis. 1987. 136:225–244.
14. Juniper EF, Frith PA, Dunnett C, Cockcroft DW, Hargreave FE. Reproducibility and comparison of responses to inhaled histamine and methacholine. Thorax. 1978. 33:705–710.
15. Park SW, Jangm HK, An MH, Min JW, Jang AS, Lee JH, Park CS. Interleukin-13 and interleukin-5 in induced sputum of eosinophilic bronchitis: comparison with asthma. Chest. 2005. 128:1921–1927.
16. Norzila MZ, Fakes K, Henry RL, Simpson J, Gibson PG. Interleukin-8 secretion and neutrophil recruitment accompanies induced sputum eosinophil activation in children with acute asthma. Am J Respir Crit Care Med. 2000. 161:769–774.
17. Pizzichini MM, Pizzichini E, Clelland L, Efthimiadis A, Mahony J, Dolovich J, Hargreave FE. Sputum in severe exacerbation of asthma: Kinetics of inflammatory indices after prednisone treatment. Am J Respir Crit Care Med. 1997. 155:1501–1508.
18. Park CS, Kim YY, Kang SY. Collection between RAST and skin test for inhalant offending allergens. Korean J Allergy. 1983. 3:1–9.
19. Cormican LJ, Farooque S, Altmannw DR, Lee TH. Improvements in an oral aspirin challenge protocol for the diagnosis of aspirin hypersensitivity. Clin Exp Allergy. 2005. 35:717–722.
20. Park JS, Chang HS, Park CS, Lee JH, Lee YM, Choi JH, Park HS, Kim LH, Park BL, Choi YH, Shin HD. Association analysis of cysteinylleukotriene receptor 2 (CYSLTR2) polymorphisms with aspirin intolerance in asthmatics. Pharmacogenet Genomics. 2005. 15:483–492.
21. Hong KY, Lee JH, Park SW, Joo JH, Kim DJ, Moon SH, Uh ST, Kim YH, Park CS, Park JS. Evaluation of emphysema in patients with asthma using high-resolution CT. Korean J Intern Med. 2002. 17:24–30.
22. Park SW, Park JS, Lee YM, Lee JH, Jang AS, Kim DJ, Hwangbo Y, Uh ST, Kim YH, Park CS. Differences in radiological/HRCT findings in eosinophilic bronchitis and asthma: implication for bronchial responsiveness. Thorax. 2006. 61:41–47.
23. Lee YM, Park JS, Hwang JH, Park SW, Uh ST, Kim YH, Park CS. High-resolution CT findings in patients with near-fatal asthma: comparison of patients with mild-to-severe asthma and normal control subjects and changes in airway abnormalities following steroid treatment. Chest. 2004. 126:1840–1848.
24. Senéterre E, Paganin F, Bruel JM, Michel FB, Bousquet J. Measurement of the internal size of bronchi using high resolution computed tomography (HRCT). Eur Respir J. 1994. 7:596–600.
25. Simons FER. Allergic rhinobronchitis: The asthma-allergic rhinitis link. J Allergy Clin Immunol. 1999. 104:534–540.
26. Church MK, Levi-Schaffer F. Updates on cells and cytokines: the human mast cells. J Allergy Clin Immunol. 1997. 99:155–160.
27. Borish L, Rosenwasser LJ. Update on cytokines. J Allergy Clin Immunol. 1996. 97:719–734.
28. Barnes PJ. Cytokines as mediators of chronic asthma. Am J Respir Crit Care Med. 1994. 150:S42–S49.
29. Braman SS, Barrows AA, De Cotiis BA, Settipane GA, Corrao WM. Airway hyperresponsiveness in allergic rhinitis. A risk factor for asthma. Chest. 1987. 91:671–674.
30. Eggleston PA. Upper airway inflammatory diseases and bronchial hyperresponsiveness. J Allergy Clin Immunol. 1988. 81:1036–1041.
31. Jeffery PK, Wardlaw AJ, Nelson FC, Collins JV, Kay AB. Bronchial biopsies in asthma:An ultrastructural, quantitative study and correlation with hyperreactivity. Am Rev Respir Dis. 1989. 140:1745–1753.
32. Lambert RK, Wiggs BR, Kuwano K, Hogg JC, Paré PD. Functional significance of increased airway smooth muscle in asthma and COPD. J Appl Physiol. 1993. 74:2771–2781.
33. Hogaboam CM, Blease K, Mehrad B, Steinhauser ML, Standiford TJ, Kunkel SL, Lukacs NW. Chronic airway hyperreactivity, goblet cell hyperplasia, and peribronchial fibrosis during allergic airway disease induced by Aspergillus fumigatus. Am J Pathol. 2000. 156:723–732.
34. Bousquet J, Jeffery PK, Busse WW, Johnson M, Vignola AM. Asthma From bronchoconstriction to airways inflammation and remodeling. Am J Respir Crit Care Med. 2000. 161:1720–1745.
35. Lazaar AL, Panettieri RA Jr. Is airway remodeling clinically relevant in asthma? Am J Med. 2003. 115:652–659.
36. Taramarcaz P, Gibson PG. The effectiveness of intranasal corticosteroids in combined allergic rhinitis and asthma syndrome. Clin Exp Allergy. 2004. 34:1883–1889.
37. Jang AS, Lee JH, Park SW, Lee YM, Uh ST, Kim YH, Park CS. Factors influencing the responsiveness to inhaled glucocorticoids of patients with moderate-to-severe asthma. Chest. 2005. 128:1140–1145.
38. Choi JS, Jang AS, Lee JH, Park JS, Park SW, Kim DJ, Park CS. Effect of high dose inhaled glucocorticoids on quality of life in patients with moderate to severe asthma. J Korean Med Sci. 2005. 20:586–590.
39. Szczeklik A, Sanak M, Nizankowska-Mogilnicka E, Kielbasa B. Aspirin intolerance and the cyclooxygenaseleukotriene pathways. Curr Opin Pulm Med. 2004. 10:51–56.
40. Samter M, Beers RF Jr. Concerning the nature of intolerance to aspirin. J Allergy. 1967. 40:281–293.
41. Szczeklik A, Stevenson DD. Aspirin-induced asthma: advances in pathogenesis, diagnosis, and management. J Allergy Clin Immunol. 2003. 111:913–921.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download