Abstract
Hyaluronidase is a goat testicular protein that hydrolyzes hyaluronic acid, a structural component of the intercellular matrix. It is commonly used as a spreading factor to improve the diffusion of drugs, including local anesthetics and chemotherapeutics. We experienced a 55-yr-old female with generalized urticaria that developed within 1 hr after the epidural injection of hyaluronidase. She had a history of allergic rhinitis, and had suffered from post-herpetic neuralgia and a herniated disc for several years. To relieve her pain, she had been given epidural injections consisting of mepivacaine hydrochloride, triamcinolone acetonide, and morphine sulfate biweekly for one year. Hyaluronidase had been administered several times with these drugs before this episode of generalized urticaria. Skin prick testing showed a positive response to 1,500 IU/mL of hyaluronidase extract, as compared to histamine. The patient's serum hyaluronidase-specific IgE level, determined using an enzyme-linked immunosorbent assay (ELISA), was markedly elevated, as compared to unexposed healthy controls. An IgE immunoblot analysis using hyaluronidase extract and the patient's serum showed IgE binding components at 31 and 21 kDa, whereas no corresponding IgE binding component was found in healthy controls. An ELISA inhibition test showed significant, dose-dependent inhibition with the serial addition of hyaluronidase extract. This is the first case of an IgE-medicated allergic reaction to goat (Naemorhedus goral raddenus) hyaluronidase, demonstrated by skin testing and a specific IgE and immunoblot assay.
Hyaluronidase is an enzyme with a temporary, reversible depolymerizing action on hyaluronic acid present in the intracellular matrix of connective tissue. It is commonly used as a spreading factor to improve the diffusion of drugs in anesthesiology, dermatology, plastic surgery, and chemotherapeutics.1,2 Occasional allergic reactions associated with hyaluronidase have been reported. The majority of cases have involved immediate hypersensitivity, such as anaphylaxis or angioedema.3,4 Rarely, delayed hypersensitivity has been reported after a peribulbar block with a combination of hyaluronidase and anesthetic in the ophthalmology literature.5 We identified a case of acute generalized urticaria caused by the injection of hyaluronidase.
A 55-yr-old woman developed generalized urticaria 1 hour after the epidural injection of 1,500 IU of hyaluronidase (H-lase®, Gunil, Seoul, Korea). She had atopy and showed a positive response to house dust mites on skin prick test. She had previously been diagnosed with allergic rhinitis, and had suffered from post-herpetic neuralgia and a herniated disc for several years. To relieve her pain, she had been given epidural injections consisting of 200 mg of mepivacaine hydrochloride (Mevan®, Hanlim, Seoul, Korea), 40 mg of triamcinolone acetonide (Triam®, Shinpung, Seoul, Korea), and 0.5 mg of morphine sulfate (Hanlim, Seoul, Korea) biweekly for one year. Hyaluronidase had been administered several times before this episode of generalized urticaria, implying that sensitization had taken place. Since she had previously been given the above-mentioned drugs without problem and had no history of allergy to anesthetics, hyaluronidase was suspected as the causative drug. Skin prick test showed a positive response to 1,500 IU/mL of hyaluronidase extract compared to that of histamine (6×4/20×19 vs. 4×3/16×15 mm).6,7 The patient's serum specific IgE antibodies to hyaluronidase were measured using an enzyme-linked immunosorbent assay (ELISA), which revealed marked elevation compared to 14 unexposed healthy controls. The positive cutoff value was determined as the mean plus three standard deviations of the absorbance values for the normal controls. An IgE immunoblot analysis using hyaluronidase extract and the patient's serum showed IgE binding components at 31 and 21 kDa, whereas no corresponding IgE binding component was found in 14 healthy controls (Fig. 1). After treatment with systemic oral steroid and antihistamines, her symptoms improved substantially. Subsequently, mepivacaine alone has been administered for the nerve block.
Allergic reactions to hyaluronidase are not common. The estimated incidence in an ophthalmic surgery department was approximately 0.1%.6 As hyaluronidase is used in various medical fields, reports on allergy to hyaluronidase have been increasing over the last decade. Recently, the epidural injection of hyaluronidase has been performed more commonly in pain clinics.8,9
In previous reports, the hyaluronidase was usually administered via local injection for peribulbar block in cataract surgery4,6 or via an intravenous route as an additive to a chemotherapeutic drug in oncology.1,3 The initial manifestations were influenced by the route of administration: when given at a local site, localized angioedema or urticaria developed;6,10,11 when given intravenously, most patients presented with anaphylactic shock, urticaria, or dyspnea.3 Our patient was administered hyaluronidase via an intrathecal route at a relatively high dose of 1,500 IU. No previous allergic reactions induced by intrathecal hyaluronidase administration have been reported.
The dosage of the drug is associated with the allergic reactions to hyaluronidase. With local reactions, the dose administered ranged from 100 to 150 IU. Patients with systemic reactions had received a single intravenous dose ranging from 1,500 to 200,000 IU. However, the cumulative dose is not related to clinical manifestations.3 Most of the patients with hyaluronidase allergy had a positive skin prick test or intradermal test.6,10 When hyaluronidase is supposed to be administrated at high-dose hyaluronidase via intravenous or intrathecal routes, skin prick test could be a useful way to predict systemic allergic reactions.
Hyaluronidase as a medical product is derived from the testes of cows, goats, and sheep. Of these, goat-derived hyaluronidase is the most widely prescribed product in Korea. In addition, hyaluronidase is present in bee and ant venoms, invasive nematodes, and group B Streptococci.1 To evaluate the cross-reactivity between hyaluronidase and honeybee and yellow jacket venoms (Phadia, Uppsala, Sweden), ELISA inhibition tests were performed. The test with hyaluronidase extract showed significant dose-dependent inhibition, whereas no inhibition was seen with the bee venom allergens.
In conclusion, the patient in this case developed generalized urticaria immediately after exposure to goat-derived hyaluronidase. A positive skin prick test and high serum-specific IgE to hyaluronidase were noted. Moreover, two IgE-binding components were identified on IgE immunoblot assay, indicating that hyaluronidase derived from the goat (Naemorhedus goral raddenus) can induce an IgE-mediated allergic reaction in exposed subjects.
Figures and Tables
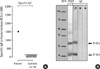 | Fig. 1Hyaluronidase-specific IgE serum levels, as determined using an enzyme-linked immunosorbent assay (ELISA), in the sensitized patient and 14 normal controls (A). Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and IgE immunoblot analysis of hyaluronidase (B) in serum from the sensitized patient (P), a healthy control (N), and a buffer control (B). |
ACKNOWLEDGMENTS
This study was supported by a grant of the Korean Health 21 R&D Project, Ministry For Health, Welfare and Family Affairs, Republic of Korea (A030001).
References
1. Girish KS, Kemparaju K. The magic glue hyaluronan and its eraser hyaluronidase: a biological overview. Life Sci. 2007. 80:1921–1943.
2. Stern R. Hyaluronidases in cancer biology. Semin Cancer Biol. 2008. 18:275–280.
3. Szepfalusi Z, Nentwich I, Dobner M, Pillwein K, Urbanek R. IgE-mediated allergic reaction to hyaluronidase in paediatric oncological patients. Eur J Pediatr. 1997. 156:199–203.
4. Escolano F, Pares N, Gonzalez I, Castillo J, Valero A, Bartolome B. Allergic reaction to hyaluronidase in cataract surgery. Eur J Anaesthesiol. 2005. 22:729–730.
5. Feighery C, McCoy EP, Johnston PB, Armstrong DK. Delayed hypersensitivity to hyaluronidase (Hyalase) used during cataract surgery. Contact Dermatitis. 2007. 57:343.
6. Leibovitch I, Tamblyn D, Casson R, Selva D. Allergic reaction to hyaluronidase: a rare cause of orbital inflammation after cataract surgery. Graefes Arch Clin Exp Ophthalmol. 2006. 244:944–949.
7. Kempeneers A, Dralands L, Ceuppens J. Hyaluronidase induced orbital pseudotumor as complication of retrobulbar anesthesia. Bull Soc Belge Ophtalmol. 1992. 243:159–166.
8. Avellanal M, Diaz-Reganon G. Interlaminar approach for epiduroscopy in patients with failed back surgery syndrome. Br J Anaesth. 2008. 101:244–249.
9. Schulze C, Bittorf T, Walzel H, Kundt G, Bader R, Mittelmeier W. Experimental evaluation of hyaluronidase activity in combination with specific drugs applied in clinical techniques of interventional pain management and local anaesthesia. Pain Physician. 2008. 11:877–883.
10. Delaere L, Zeyen T, Foets B, Van Calster J, Stalmans I. Allergic reaction to hyaluronidase after retrobulbar anaesthesia: a case series and review. Int Ophthalmol. 2008.
11. Andre P, Flechet ML. Angioedema after ovine hyaluronidase injection for treating hyaluronic acid overcorrection. J Cosmet Dermatol. 2008. 7:136–138.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download