Abstract
Objectives
Phytoestrogen-containing pulse supplements have beneficial effects on postmenopausal symptoms, but how such effects are achieved is unclear. This study investigates the effects of pulse consumption on the menopausal changes in ovariectomized rats.
Methods
Female Sprague-Dawley rats were either sham operated (Sham; n = 3) or surgically ovariectomized (n = 13). The Sham group was fed the regular AIN-93M diet. Ovariectomized group was divided into 3 sub-groups and fed AIN-93M containing soybean (n = 5), mung bean (n = 3), or cowpea (n = 5) for 10 weeks. At the end of the experiment, all rats were sacrificed, and the uterus was harvested, rinsed, and weighed. Expressions of vitamin D receptor (VDR), estrogen receptor (ER) β, and ezrin in uterus were evaluated by immunohistochemistry.
Results
VDR was highly expressed in the uterus of rat, irrespective of ovariectomized state. VDR was more definitely expressed in the uterus of ovariectomized groups than the sham-operated group. There were no significant differences in expression of ER β. However the expression of ezrin was highly expressed in the cowpea group compared to sham group (P = 0.044).
Conclusion
This study suggested that legumes diet may concern menopausal changes via VDR and ezrin. The result may partly explain the beneficial effects of VDR on menopausal symptoms. Further study is necessary to study the detailed mechanisms of VDR and ezrin on the menopausal changes in the uterus.
Figures and Tables
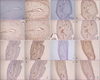 | Fig. 1Localization of vitamin D receptor, ezrin, and estrogen beta receptor in the uterus of rats. Immunohistochemistry was performed as described in the Material and Methods section. (A) Sham control, (B) OVX-SB, (C) OVX-MB, (D) OVX-CP. A negative control shows any immunoreacitivity without a primary antibody treatment. 1: negative control, 2: vitamin D receptor, 3: ezrin, 4: Estrogen receptor beta. Magnification: × 100. OVX-SB: ovariectomized and the intake of soybean, OVX-MB: ovariectomized and the intake of mung bean, OVX-CP: ovariectomized and the intake of cowpea. |
 | Fig. 2Immunoreacitivity of vitamin D receptor, estrogen receptor and ezrin in rat's uterus were measured by Image J. Semiquantitative intensity of immunoreacity in sham-operated rats (Sham) and ovariectomized rats (OVX) were compared. |
 | Fig. 3Immunoreacitivity of VDR, ER, and ezrin in rat's uterus were measured by Image J. Imunoreacitive ezrin expression was significantly lower in OVX-MB group than Sham control group (t-test, *P = 0.04). VDR: vitamin D receptor, ER: estrogen receptor, OVX-SB: ovariectomized and the intake of soybean, OVX-MB: ovariectomized and the intake of soybean, OVX-CP: ovariectomized and the intake of soybean. |
References
1. Anderson JW, Smith BM, Washnock CS. Cardiovascular and renal benefits of dry bean and soybean intake. Am J Clin Nutr. 1999. 70:464S–474S.
2. Messina MJ. Legumes and soybeans: overview of their nutritional profiles and health effects. Am J Clin Nutr. 1999. 70:439S–450S.
3. Chen YM, Ho SC, Lam SS, Ho SS, Woo JL. Soy isoflavones have a favorable effect on bone loss in Chinese postmenopausal women with lower bone mass: a double-blind, randomized, controlled trial. J Clin Endocrinol Metab. 2003. 88:4740–4747.
4. Chiechi LM, Secreto G, D'Amore M, Fanelli M, Venturelli E, Cantatore F, et al. Efficacy of a soy rich diet in preventing postmenopausal osteoporosis: the Menfis randomized trial. Maturitas. 2002. 42:295–300.
5. Harkness L. Soy and bone. Where do we stand? Orthop Nurs. 2004. 23:12–17.
6. Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc. 2006. 81:353–373.
7. Lips P. Vitamin D physiology. Prog Biophys Mol Biol. 2006. 92:4–8.
8. Mosselman S, Polman J, Dijkema R. ER beta: identification and characterization of a novel human estrogen receptor. FEBS Lett. 1996. 392:49–53.
9. Enmark E, Pelto-Huikko M, Grandien K, Lagercrantz S, Lagercrantz J, Fried G, et al. Human estrogen receptor beta-gene structure, chromosomal localization, and expression pattern. J Clin Endocrinol Metab. 1997. 82:4258–4265.
10. Tremblay GB, Tremblay A, Copeland NG, Gilbert DJ, Jenkins NA, Labrie F, et al. Cloning, chromosomal localization, and functional analysis of the murine estrogen receptor beta. Mol Endocrinol. 1997. 11:353–365.
11. Heldring N, Pike A, Andersson S, Matthews J, Cheng G, Hartman J, et al. Estrogen receptors: how do they signal and what are their targets. Physiol Rev. 2007. 87:905–931.
12. Hess RA. Estrogen in the adult male reproductive tract: a review. Reprod Biol Endocrinol. 2003. 1:52.
13. McDonnell DP, Chang CY, Norris JD. Capitalizing on the complexities of estrogen receptor pharmacology in the quest for the perfect SERM. Ann N Y Acad Sci. 2001. 949:16–35.
14. Kuiper GG, Gustafsson JA. The novel estrogen receptor-beta subtype: potential role in the cell- and promoter-specific actions of estrogens and anti-estrogens. FEBS Lett. 1997. 410:87–90.
15. Hiscox S, Jiang WG. Ezrin regulates cell-cell and cell-matrix adhesion, a possible role with E-cadherin/beta-catenin. J Cell Sci. 1999. 112(Pt 18):3081–3090.
16. Curto M, McClatchey AI. Ezrin...a metastatic detERMinant? Cancer Cell. 2004. 5:113–114.
17. Athanasopoulou A, Aroukatos P, Nakas D, Repanti M, Papadaki H, Bravou V. Decreased ezrin and paxillin expression in human urothelial bladder tumors correlate with tumor progression. Urol Oncol. 2011.
18. Khanna C, Wan X, Bose S, Cassaday R, Olomu O, Mendoza A, et al. The membrane-cytoskeleton linker ezrin is necessary for osteosarcoma metastasis. Nat Med. 2004. 10:182–186.
19. Hunter KW. Ezrin, a key component in tumor metastasis. Trends Mol Med. 2004. 10:201–204.
20. Song J, Fadiel A, Edusa V, Chen Z, So J, Sakamoto H, et al. Estradiol-induced ezrin overexpression in ovarian cancer: a new signaling domain for estrogen. Cancer Lett. 2005. 220:57–65.
21. Smith PM, Cowan A, Milgram SL, White BA. Tissue-specific regulation by estrogen of ezrin and ezrin/radixin/moesin-binding protein 50. Endocrine. 2003. 22:119–126.
22. Baeza I, De Castro NM, Giménez-Llort L, De la Fuente M. Ovariectomy, a model of menopause in rodents, causes a premature aging of the nervous and immune systems. J Neuroimmunol. 2010. 219:90–99.
23. Zarnani AH, Shahbazi M, Salek-Moghaddam A, Zareie M, Tavakoli M, Ghasemi J, et al. Vitamin D3 receptor is expressed in the endometrium of cycling mice throughout the estrous cycle. Fertil Steril. 2010. 93:2738–2743.
24. Adlercreutz H, Hämäläinen E, Gorbach S, Goldin B. Dietary phyto-oestrogens and the menopause in Japan. Lancet. 1992. 339:1233.
25. Marini H, Minutoli L, Polito F, Bitto A, Altavilla D, Atteritano M, et al. Effects of the phytoestrogen genistein on bone metabolism in osteopenic postmenopausal women: a randomized trial. Ann Intern Med. 2007. 146:839–847.
26. Roach VJ, Cheung TF, Chung TK, Hjelm NM, Waring MA, Loong EP, et al. Phytoestrogens: dietary intake and excretion in postmenopausal Chinese women. Climacteric. 1998. 1:290–295.
27. Pan Y, Anthony MS, Binns M, Clarkson TB. A comparison of oral micronized estradiol with soy phytoestrogen effects on tail skin temperatures of ovariectomized rats. Menopause. 2001. 8:171–174.
28. Lu LJ, Tice JA, Bellino FL. Phytoestrogens and healthy aging: gaps in knowledge. A workshop report. Menopause. 2001. 8:157–170.
29. Song AR. A study on management of menopause of climacteric women. J Korean Soc Menopause. 1997. 3:140–160.
30. Knight DC, Howes JB, Eden JA, Howes LG. Effects on menopausal symptoms and acceptability of isoflavone-containing soy powder dietary supplementation. Climacteric. 2001. 4:13–18.
31. Lee SK. Study on the impact of isoflavone in hormonal profiles during the menstrual cycle and in the postmenopausal parameters in Korean women [Doctoral Dissertation]. 2001. Seoul: Yonsei University.
32. Adams MR, Golden DL, Register TC, Anthony MS, Hodgin JB, Maeda N, et al. The atheroprotective effect of dietary soy isoflavones in apolipoprotein E-/- mice requires the presence of estrogen receptor-alpha. Arterioscler Thromb Vasc Biol. 2002. 22:1859–1864.
33. Lichtenstein AH. Approaches to reducing cardiovascular disease risk: food or pills? Curr Opin Lipidol. 2001. 12:1–3.
34. Alekel DL, Germain AS, Peterson CT, Hanson KB, Stewart JW, Toda T. Isoflavone-rich soy protein isolate attenuates bone loss in the lumbar spine of perimenopausal women. Am J Clin Nutr. 2000. 72:844–852.
35. Francis RM, Boyle IT, Moniz C, Sutcliffe AM, Davis BS, Beastall GH, et al. A comparison of the effects of alfacalcidol treatment and vitamin D2 supplementation on calcium absorption in elderly women with vertebral fractures. Osteoporos Int. 1996. 6:284–290.
36. Yildirim B, Kaleli B, Düzcan E, Topuz O. The effects of postmenopausal vitamin D treatment on vaginal atrophy. Maturitas. 2004. 49:334–337.
37. Somjen D, Kohen F, Gayer B, Knoll E, Limor R, Baz M, et al. A non-calcemic vitamin D analog modulates both nuclear and putative membranal estrogen receptors in cultured human vascular smooth muscle cells. J Steroid Biochem Mol Biol. 2004. 89-90:397–399.
38. Nashold FE, Spach KM, Spanier JA, Hayes CE. Estrogen controls vitamin D3-mediated resistance to experimental autoimmune encephalomyelitis by controlling vitamin D3 metabolism and receptor expression. J Immunol. 2009. 183:3672–3681.
39. Cavallini A, Dinaro E, Giocolano A, Caringella AM, Ferreri R, Tutino V, et al. Estrogen receptor (ER) and ER-related receptor expression in normal and atrophic human vagina. Maturitas. 2008. 59:219–225.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download