Abstract
Ezrin, a membrane cytoskeleton linking protein, is a member of ezrin/radixin/moesin (ERM) that regulates cell shape, motility and cell to cell interaction via linking the contractile elements of the cell to transmembrane proteins. Ezrin, through this mechanism, has been thought to play an important role in cancer progression and distant metastasis. In addition, high levels of ezrin expression have been noted in many cancers, such as breast, colon, osteosarcoma, and prostate cancer. Gynecologic cancer cells, with high levels of ezrin expression, have more invasive potential than that of the lower levels of ezrin expressed cancer cells. High levels of ezrin expression are also related to the advanced histological grade and poor outcome. Recently, several reports have also demonstrated that ezrin expression is enhanced and almost localized at the membranous portion in high stage tumor cells and metastatic gynecologic cancer cells. Therefore, in the near future, ezrin levels and its cellular location might serve as essential markers for the metastasis of gynecologic cancers.
Worldwide, gynecologic cancers account for approximately 22.9% of all female cancers, second only to breast cancer.1 In 2004, approximately 73,000 women in the United States were diagnosed with a cancer affecting the reproductive organs, and approximately 27,000 women died from gynecologic cancer. Among the different types of gynecologic cancer, endometrial cancer is the most common while ovarian cancer is the most deadly. It is ranked the fifth most common cause of cancer deaths in women.2 This condition is strongly related to late detection and increased prevalence in older women.
Furthermore, in spite of having advanced diagnostic tools for gynecologic cancer detection and improved treatment modalities, there is no outstanding achievement of survival rate in patients with gynecologic cancer, and a lot of cases of advanced gynecologic malignancies are resistant to chemotherapy.3 So, many researchers have focused on the basic mechanism of cancer progression and distant metastasis to find clues that will improve the efficacy of cancer treatment.
Many researchers are trying to find key proteins that are relevant in transforming normal cells to dysplastic or cancer cells. These proteins can better explain the complicated process of distant metastasis of cancer cells.4 A specific characteristic of cancer cells that is particularly relevant includes the ability to survive and proliferate continuously even under poor conditions. In order to achieve this, cancer cells must be in constant communication with normal cells as well as other cancer cells. Furthermore, to successfully metastasize at significant distances and survive, cancer cells should have the ability to break the linkages between cells or between the cells and the matrix, penetrate the endothelium, migrate to other sites without mishap, and successfully attach to new sites.5
Among the various proteins that researchers are focusing on, ezrin/radixin/moesin (ERM) proteins are receiving the most attention because they can help explain the functional and structural communication needed between normal cells and cancerous cells in the sequential process of cancer progression.6,7
Ezrin is a member of the ERM protein family that acts both as an adherens junction between transmembranous proteins and the cytoskeleton and as a signal transducer. In this way, ezrin is involved in the regulation of phenotypical changes in cellular architecture, controlling cellular motility, tethering action between cell to cell and cell to matrix, recognizing internal or external signal and conducting cellular growth and invasion above mentioned. All of these cellular functions are essential for maintaining homeostasis of normal cells as well as the entire process of tumorigenesis and distant metastasis.8
Recently, using tissue microarray, ezrin expression was found in most cancers and normal tissues at varying levels of intensity. Abnormal or increased expression of ezrin was associated with immortalization and malignant transformation of mouse fibroblasts.9 It also has been reported that high levels of ezrin expression is implicated high invasiveness of tumor cells and poor prognosis in several animal and human malignancies such as carcinoma of the endometrium,10 pancreas, prostate, uveal melanoma, and astrocytoma. More specifically, ezrin was expressed at higher levels in sarcomas than in carcinomas. By normalizing the expression of ezrin in each cancer using ezrin expression found in the corresponding normal tissue, significant associations between ezrin and advancing histological grade in sarcomas and poor outcome in breast cancer were found.11
In addition to ezrin overexpression in cancer cells, the cellular localization of ezrin is observed in a different pattern; ezrin is localized at the apical membrane in normal cells; however, it is distributed throughout the cytoplasmic or membranous portion in cancer cells. Ezrin is mostly found in the membranous portion of metastatic cancer cells, though.10,12,13 The differences in the localization of ezrin expression may imply that ezrin plays a role in the process of distant metastasis of cancer cells.
Estrogen, in particular, regulates ezrin expression on the genomic basis and controls functional activity by adjusting the synthesis of co-activating proteins.
Given ezrin's linking and signal transduction ability in addition to its regulation by estrogen, it is quite reasonable to examine ezrin in the gynecologic area. Therefore, this paper aims to review the effects of ezrin on the metastasis of commonly estrogen dependent gynecologic cancer. Ultimately, we hope to encourage research examining the levels and cellular location of ezrin as markers for the metastasis of gynecologic cancer.
In normal cells and tissues, the distribution of ezrin is confined to the apical portion of microvilli. It maintains cellular polarity and forms a specific cellular shape to accomplish its specific missions. Ezrin also contributes to the maintenance of the normal shape of tissues by binding actin filaments that can fortify the intercellular adhesion.6
However, in the process of cancer progression, ezrin expression is intensified and cellular redistribution of ezrin to the cytoplasm is common.10,13 This may imply that the dormant forms of ezrin increase more than the active forms, which decreases the tethering force between the cells and enables them to detach from neighboring cells more easily than under normal conditions. Increased dormant ezrins also cause the ruffle border to disappear.
In addition to these effects, intensified ezrin expression can fortify cellular invasiveness that is thought to be an essential factor for early metastatic events. For example, ezrin was significantly overexpressed in highly metastatic murine rhabdomyosarcoma and osteosarcoma cell lines relative to their poorly metastatic counterparts. By suppressing ezrin protein expression or disrupting ezrin phosphorylation, the metastatic capability of both cell lines was reduced. The highly metastatic osteosarcoma cells with decreased ezrin expression that reached the lung did not survived after 24 hours. In contrast, cells with ezrin were viable and had longer cellular processes.14 These facts imply that overexpressed ezrin makes metastatic cancer cells achieve a stronger power of invasiveness and active forms of ezrin concentrated in the membranous portions of cells can make a strong attachment to distant metastatic sites. Therefore, the expression of ezrin and its location in the cell can be a strong marker for the metastasis of cancer (Fig. 1).
Ezrin is composed of 585 amino acids with three distinctive regions. The highly conserved N-terminal ERM associated domain (N-ERMAD) contains 296 amino acids. It is followed by an extended α- helix and a positively charged C-ERMAD that contains 107 amino acids.15
All ERM proteins have structures similar to ezrin. Their N-terminal domains bind to plasma membranes and their C-terminal domains bind to actin filaments. The N-terminal domain of ezrin has 84%, 83%, and 62% homology with other ERM proteins, radixin, moesin and merlin respectively. The globular band 4.1, ERM (FERM) domain of the N-ERM proteins is composed of three subdomains that are arranged like a clover leaf.6
Ezrin can exist as one of two forms in the cytoplasm, active or dormant: After leaving the Golgi complex, it remains in the perinuclear area of the cytoplasm until it is activated. The active form exposes the binding domain of both terminals and is currently thought to be associated with specific cellular function. Since the N-ERMAD of ezrin can be associated with the C-ERMAD of ezrin, Pearson et al. confirmed that it is possible that a conformational change could mask the binding sites of both terminals, which would inactivate the ezrin molecule. The conformational change of ezrin to its activated form is fundamental to accomplishing its specific cellular actions. After changing into its active form, it moves promptly to the membranous portion to bind to specific molecules that transduce cellular signaling and maintain cellular scaffolding in its normal condition. However, in cancer cells, ezrin is usually localized in the cytoplasm where it is most often in its dormant form.15 When ezrin is in its dorman form, cancer cells can detach from primary sites more easily (Fig. 2). Therefore, an increase of dormant ezrin in the cytoplasm could be used as a marker for metastatic cancer.
The cytoskeleton is the cellular scaffolding contained within the cytoplasm of all cells. It was once thought that this structure was unique to eukaryotes, but recent research has identified prokaryotic cytoskeletons. Since the concept of a cytoskeleton was first introduced by Paul Wintrebert, many studies have examined its roles in cellular function.16 The cytoskeleton is a dynamic structure that maintains cell shape, protects the cell, enables cellular motion (using structures such as flagella, cilia and ruffles), and plays important roles in both intracellular transport and cellular division. Around 6 nm in diameter, this filament type is composed of two intertwined actin chains. The cytoskeleton is most concentrated just beneath the cell membrane and is responsible for resisting tension, forming cytoplasmatic protuberances and participating in some cell-to-cell or cell-to-matrix tethering.17 Breaking the tethering between cells and moving forward are the first steps towards successful local invasion or distant metastasis of cancer cells. Although other coactivating factors are needed, the actin cytoskeleton is considered essential for pushing the cell forward in the surrounding environment. The cytoskeleton can dynamically reconstruct cell shape and continuously polymerize actin. Furthermore, it is widely accepted that the polymerization of actin to generate protrusive activity at the front coupled with the contractile shortening of membrane anchored actin-myosin filaments leads to propulsion of the cell body forward. In contrast, the rear of the cell detaches from the substrate and retracts, which generates definitive movement in the direction of migration.18 This rearrangement of the cytoskeleton leads to changes in cell shape that allows it to move to other sites more easily. It is achieved with the help of a number of accessory proteins and signaling pathways that mediate growth factors and cytokine signals.
Sex steroids are involved in recruiting G proteins, tyrosine kinase, c-Src, phosphatidylinositol 3-kinase/Akt (PI3K/Akt), and mitogen activated protein kinases. Once one or more of these molecules is recruited, actin is rapidly activated, which leads to cell remodeling and motility. In human endothelial cells, physiological levels of estrogen lead to a rapid remodeling of the actin cytoskeleton and the formation of a cortical actin complex.
ERM proteins, which are also under the control of estrogen, participate in formatting ruffles border and pseudopodia of the cells too. In addition, they play pivotal roles in cellular motility via actin-binding function.14 Given ERM proteins' involvement in cytoskeleton adjustment and their relationship to estrogen, it would be logical to study them as markers for metastasis.
Ezrin was first identified as a linker protein between the plasma membrane and cytoskeleton. The N-terminal of ERM proteins, including ezrin, attaches to the C-terminal of integral proteins in the plasma membrane directly or indirectly.19
CD44 is a well known counter binding membranous protein of ezrin and is highly expressed in various human cells.20 CD44 and CD44v4, its variant, are positively stained in primary ovarian cancer and metastatic ovarian cancer. They are also associated with the migration of tumor cells to specific sites of metastasis formation in many cancers involving gynecologic cancer. Ezrin binds to the C-terminal ends of CD44 and influences its function as a tumor promoter. Some growth factors promote this action and intensify its role for cellular proliferation and invasiveness.21
Another contributing factor for tumor cell invasion, osteopontin (OPN), is co-localized in the integral parts of the CD44 and ezrin complex. OPN promotes ovarian cancer progression, cell survival and metastasis. So, ezrin may be involved in the process of cancer cell invasion indirectly via plasma proteins and related factors.
Ezrin also can directly bind to intercellular adhesion molecules, such as Intercellular adhesion molecule-1 (ICAM-1) and Intercellular adhesion molecule-2 (ICAM-2), which are colocalized with ezrin in microvilli projections and interact with the cytoskeletal protein α-actinin. Intercellular ICAM-1 mediates many adhesion dependent cell-cell interactions. ICAM-1 expression can be induced by inflammatory cytokines such as interleukin (IL)1-β and tumour necrosis factor (TNF)-α in non-hemopoietic cells including vascular endothelium, thymic epithelium and dermal fibroblasts.22 ICAM-1 expression was decreased in ovarian adenocarcinoma cell lines compared with the immortalized human ovarian surface epithelial (HOSE) cells at both the RNA and protein levels.23 Few studies have examined ICAM-1 and gynecologic cancer, but a decreased level of ICAM may be induced by the increased dormant form of ezrin in the cytoplasm.
ICAM-2, another ezrin binding molecule, mediates adhesive interactions important for antigen-specific immune responses, such as lymphocyte recirculation, natural killer (NK)-cell mediated clearance, and other cellular interactions important for immune responses and surveillance.24 The killing activity of NK cells associated with IL-2-activated depends on the distribution of ICAM-2. The level of ICAM-2 expression in NK-sensitive cells and NK-resistant cells is similar, but in sensitive cells ICAM-2 is concentrated into bud-like cellular projections known as uropods, whereas in resistant cells it is evenly distributed. The cytoskeletal-membrane linker protein ezrin is also localized in uropods. Transfection of human ezrin into NK-resistant cells induces uropod formation, redistribution of ICAM-2 and ezrin, and sensitizes target cells to IL-2-activated killing.24 Based on these observations, dormant ezrin commonly observed in cytoplasm may confer the tumor cells the chance to escape immune surveillance in the process of tumorigenesis by even distribution of ICAM-2. These results are concordant with our hypothesis that ezrin expression moves from the apex to cytoplasm and changes into its dormant state in early stage of metastasis, which may results in increased survival of cancer cells against host immune system. Therefore, ezrin localization in the cell may be an important determination factor for the tumor cell survival relating host immune system.
ERM-binding phosphoprotein 50 (EBP50), another important binding protein of the ERM family, was found in human placenta and the bovine brain.25 EBP50 binds tightly to the N-ERMADs of ezrin. It has two postsynaptic density protein 95 (PSD-95)/drosophila disc large tumour suppressor (DlgA)/zonula occludens (ZO)-1-like (PDZ) domains that can associate with the C-terminal of integral transmembranous proteins. The C-terminal of ERM proteins also can bind to filamentous actin (F-actin).25 Therefore, EBP50 can mediate links between membranous proteins and ERM proteins, and interestingly, both EBP50 and ezrin are controlled by estrogen tissue specifically.26 Recently, an elevated accumulation of EBP50 protein was readily detected in the cytoplasm compared with levels in the surrounding non-cancerous epithelial cells in breast carcinomas. In morphologically normal epithelial cells, EBP50 was stained mostly at apical membranous portion and EBP50 immunoreactivity was significantly associated with the tumor stage, lymph node and estrogen receptor (ER) status. Depending on its intracellular distribution, EBP50 may behave either as a tumor suppressor when it is localized at the plasma membrane or as an oncogenic protein, when it is shifted to the cytoplasm.27 These results may also be due to decreased active ezrin; increased dormant forms of ezrin lead to move EBP50 from apex to cytoplasm, and the counterpart proteins can be easily detached from their binding sites. So, the detachment of cancer cell from neighboring cells may be possible in the early stage of metastasis due to changes in ezrin concentration and location (Fig. 3).
A large part of ezrin found in the cytoplasm is in its inactive form and must be activated to participate in cellular reactions. Several activation mechanisms have been proposed and confirmed. These mechanisms are important to understand because they affect ezrin's location and activation status. First, specific residues of the ERM protein, such as Tyr145 and Tyr353, are phosphorylated in vitro and in vivo by the epidermal growth factor (EGF) receptor. Hepatocyte growth factor (HGF) also can phosphorylate these sites under specific conditions.28
Second, serine/threonine residues are other important phosphorylating sites of ERM proteins. Dephosphorylation of these sites results in ezrin translocation from the plasma membrane to the cytoplasm, disappearance of microvilli and disruption of the renal brush border.29 A threonine residue of C-terminal can also be phosphorylated in moesin and radixin.
Binding to phosphatidylinositol is another activation method. ERM proteins can be linked to the cytoplasmic tail of membrane proteins with a single transmembrane domain. Some of these reactions are more easily developed under the phosphatidylinositol 4, 5-bisphosphate (PIP2) existing condition.30 ERM proteins can directly interact with PIP2 containing phospholipids layers. Cytoplasmic domains of CD44 can bind to full lengths of ezrin through phosphatidylinositol phosphate (PIP) or PIP2 in vitro.30 In fact, ezrin contains two PIP2 binding sites on amino acids 12-115 and 233-310 at the N-terminal.31
Multiple mechanisms are involved in the activation and deactivation of ezrin, and this process is important for determining ezrin's location in the cell. This classification is key since in certain places and forms ezrin may help cancer cells detach from primary sites and survive under host immune surveillance in the initial process of metastasis (Fig. 2).
It is known that the proteins of the Rho GTPase family regulate all the characteristics of tumor cells, such as uncontrolled proliferation, loss of polarity, altered interactions with neighboring cells and the surrounding extracellular matrix, and enhanced migratory properties.32 For that reason, the Rho GTPase family has been extensively studied in the regulation of cytoskeletal dynamics, gene transcription, cell cycle progression, and cell transformation and distant metastasis. The genes encoding Rho proteins are known as proto-oncogenes since when properly mutated they can induce tumorigenic cell transformation. Rho proteins also play a role in the metastatic phenotype.33
The Rho pathway is thought to be one of the main mechanisms of signal transduction for activation of ERM proteins. Rho is activated by various internal or external factors. The small GTPases of the Rho family, like RhoA, Rac1, and CDC42, act as molecular switches, cycling between an active GTP-bound state and an inactive guanosine diphosphate (GDP)-bound state. These Rho GTPases are under the control of guanine nucleotide exchange factors (GEFs) and GTPase activating proteins (GAPs). GEFs catalyze the conversion to the GDP-bound state, and GAPs accelerate the inverse reaction. ctivated Rho protein causes dormant ERM proteins to become active via phosphatidylinositol-4-phosphate 5-kinase (PIP5K), which increases the amount of PIP2. As a result, increased PIP2 binds to the N-terminal of ERM proteins, promoting the unfolding of latent ERM proteins, which exposes the protein's interacting sites.34 Interestingly, the activated ERM proteins can also activate Rho. Therefore, ezrin can lead to the displacement of Rho guanine nucleotide dissociation inhibitors (RhoGDIs), a inhibitor of Rho activation, from the RhoGDP-GDI complex that results in activation of Rho.35 Therefore, ezrin may act as an enhancer for metastasis of ovarian cancer (Fig. 3).
It is well known that estrogen also affects signal transduction. It activates several membranous or cytoplasmic kinase cascades, including the PI3K/Akt cascade, a signaling pathway that plays a key role in cell survival and apoptosis. It has been studied as a mechanism involved with cancer progression and metastasis. It plays a pivotal role in growth, proliferation, and anti-apoptotic mechanisms that promote cell cycling and survival not only in normal cells but also in a variety of tumor cells. Therefore, it is not surprising that ezrin, which is also controlled by estrogen, participates in the activation of Akt. Akt, in particular, is a key factor for cell survival because it receives signals from the outside of the cell for the first time and transfers them to the intracellular system. This process involves regulating protein synthesis, the apoptotic process, proliferation, glucose metabolism and lots of other functions needed for cell survival. Akt is activated by PI3K activity, because Akt requires the formation of the phosphatidylinositol 3, 4, 5 trisphosphate (PIP3) molecule in order to be translocated to the cell membrane. With PIP3, Akt can be phosphorylated by another kinase called phosphoinositide dependent protein kinase 1 (PDK1). Class I PI3K, among the three different classes of PI3Ks, converts PIP2 to PIP3 which activates Akt. Class I PI3Ks are composed of a catalytic subunit known as p110 and a regulatory subunit either related to p85 or p101. The p85 subunits contain Src homology 2 (SH2) domain and Src homology 3 (SH3) domains.
It was reported that ezrin participates in the breast cancer motility and invasion stages of the metastatic process via c-Src and PI3K/Akt. Therefore, ezrin is thought to participate in parts of cellular signal transduction as a transducer in the distant metastatic process of gynecologic cancer, so it is quite reasonable to consider it as a marker for distant metastasic gynecologic cancer.
The small GTPases of the Rho family act as molecular switches, cycling between an active GTP-bound state and an inactive GDP-bound state, a process that is regulated by GEF and GAP. GEF catalyze the conversion to the GTP-bound state and GAP accelerates the intrinsic rate of hydrolysis of bound GTP to GDP. Rho associated protein kinase (ROCK) can also phosphorylate the sodium-hydrogen exchanger. Sodium-hydrogen exchanger 1 (NHE1), on the other hand, interacts with ERM proteins. ERM Proteins can also be activated by Rho via PIP5K. Interestingly, after the ezrin activated, it can also activate the Rho. Both Rac and Rho activate PIP5K, which increases the amount of PIP2, PIP2 then activates ERM proteins by inhibiting their interdomain interaction, which allows phosphorylation of their carboxy-terminal threonine by some kinases. Threonine-phosphorylated carboxy-terminal of ERM proteins are stabile in their activated forms. It functions as an actin filament/plasma membrane cross-linker to form microvilli. Activated ERM proteins are associated directly with adhesion molecules such as CD44 and ICAM-1, -2, and indirectly with other integral membrane proteins such as NHE3 through EBP50/sodium-hydrogen exchanger regulatory factor (NHERF) (Fig. 4).
The first evidence of estrogen's (estradiol-17β [E2]) regulation of ezrin was demonstrated in pituitary GH3 cells. The investigators observed that E2 stimulates ezrin gene expression by using a cDNA expression array and confirmed that ezrin mRNA was increased by E2, but the level of ezrin mRNA was decreased by antiestrogen. They also found that E2 elevated ezrin protein levels in whole-cell lysates and in the cytoskeleton-associated, detergent-insoluble fraction. Furthermore, ezrin was associated with free apical membranes of E2-treated cells.36 This means that E2 regulates the ezrin gene expression as well as its activation. Using differential display RNA methods, Ediger et al. have identified the human homolog of the NHERF as being under rapid and direct regulation of estrogen in the ER-containing breast cancer cells.
Recently, it was reported that there is tissue specificity in the expression of ezrin and EBP50 by estrogen. Another study noted that ezrin and EBP50 expression is coordinately increased by E2 in GH3 cells and rat pituitary glands. Ezrin levels are repressed by the steroidal antiestrogen, and reversed by E2 and the ERα-specific agonist in GH3 cells, but EBP50 levels remained constant during these treatments. Ezrin and EBP50 did not display extensive colocalization. In juvenile female rats, E2 injections increased ezrin expression in the pituitary and uterus, but increased EBP50 expression was observed only in the uterus.26 Another study revealed that NHERF expression was markedly increased in the cytoplasm, luminal membrane of glandular epithelium and stromal cells of proliferative endometrium, but only weakly expressed in the secretory endometrium. Furthermore, ER status and NHERF expression correlates closely in breast carcinoma specimens.37 Therefore, estrogen is involved with ezrin gene expression and activation directly or indirectly. It also affects where ezrin is located in the cell. The ezrin controlled by estrogen also could be involved in the process of metastasis via various mechanisms such as, influencing intercellular or intracellular linkers, signal transducers, oncogene activation and suppression of tumor suppression genes. As a result of this relationship between estrogen and ezrin, it is quite reasonable for ezrin to be a strong candidate marker for metastasis of gynecologic cancers.
Since it was first reported that ezrin is implicated in the invasion of endometrial cancer cells,38 many researchers have continued to examine the role of ezrin in the tumor invasion of many other cancer cells.
In one study of ezrin expression and translocation in ovarian cancer, investigators observed the changes of ezrin distribution in the cells and its phenotypical change after IL-1α or EGF treatment in a normal ovary, primary epithelial ovarian carcinoma (OVCA), metastatic OVCA tissue and three different ovarian cancer cell lines. They confirmed that ezrin expression is increased in metastatic OVCA and ascites cells, primary OVCA, and normal ovaries. The highest levels of ezrin were observed in the metastatic tissue and cells. In the IL-1α and EGF untreated SKOV3 cells, the cells were smooth and rounded without membrane ruffling, had very few protrusions, and ezrin was distributed evenly in cytoplasm and along the margin of the cells. However, IL-1α and EGF also enhanced OVCA cell proliferation and induced ezrin translocation, tyrosine phosphorylation, process formation, and membrane ruffling in SKOV3 cells. Nevertheless, all of these effects induced by IL-1α and EGF were abolished by Genistein, a specific protein tyrosine kinase (PTK) inhibitor.12
Another experimental study was conducted to evaluate the reciprocal action of estrogen and ezrin on ovarian cancer cells. The researchers administrated 17β-E2 to SKOV3 (ER α dominant) and DOV13 OVCA (ER β dominant) cell lines. They found that E2 induced ezrin over-expression and an invasive phenotype where ezrin was translocated to cell edges, pseudopodia and membrane ruffles. E2, in a dose-related manner, also increased cell number and enhanced OVCA cell proliferation, motility and increased its ability to penetrate Matrigel. In addition to these effects, they observed that there exists a clear positive correlation between ezrin expression and matrigel penetration ability. However, raloxifene or tamoxifen, an ER blocker, blocked all of these estrogenic effects. Therefore, these experiments indicate that the effects of estrogen on OVCA growth and phenotypic changes are in part due to the induction of ezrin over-expression, a process ERs are also strongly involved in.39 Since ezrin expression is increased with cancerous cellular severity, its location moves to membranous portion and it is accompanied by proper phenotypical changes that allow for successful metastasis, ezrin levels and its location could be used to monitor the process of metastasis.
Although the ezrin encoding gene is up regulated in many estrogen dependent tumors, until recently there has been no precise evidence that ezrin is involved in tumor progression or invasion of commonly estrogen dependent gynecologic tumors. Only recently was a study published that reported that ezrin contributes to at least the migration and invasiveness of endometrial cancer cells. The researchers investigated ezrin expression and its invasive ability in two human endometrial cancer cell lines (Ishikawa, low-metastatic endometrial cancer cell line, and its subclone [mEIIL] with high-metastatic activity and higher ezrin expression). Using the matrigel invasion assay, they estimated the change of cellular invasiveness after treating cells with ezrin antisense phosphorothioate oligonucleotids (ePONs) for the purpose of blocking ezrin, moesin, and other related proteins such as, spectrin, α-catenin, even though only ezrin was selectively affected by ePONs.
In both groups, matrigel penetrated cells were significantly decreased after treatment with ePONs, but cell proliferation was not affected and ezrin expression was inhibited by ePONs at the protein level. mEIIL cell lines with high ezrin expression were better able to migrate through the Matrigel membrane compared with Ishikawa cell lines.38
In addition, another study provided direct evidence of ezrin expression in normal human endometrial tissues and in endometrial adenocarcinoma. It evaluated the expression of ezrin according to the series of development of endometrioid adenocarcinoma progression, from normal to hyperplasia and adenocarcinoma, involving metastatic tumor. Ezrin expression was progressively increased according to aggressive disease progression. Ezrin was expressed abundantly in atypical endometrial hyperplasia and adenocarcinoma compared with normal endometrium, simple endometrial hyperplasia and complex endometrial hyperplasia. Furthermore, ezrin was expressed significantly more in metastatic lesions compared with primary lesions. These results are consistent with a previous report that ezrin expression was higher in strong metastatic potential cell lines than in weak metastatic potential endometrial cancer cell lines. Cell distribution of ezrin was analyzed by immunohistochemistry. Ezrin was localized in the membranous portions of metastasized cancer cells, whereas ezrin was mainly distributed in the cytoplasm of most cancer cells and some endometrial hyperplasia cells. Similarly, western blots showed that ezrin was distributed throughout the membranous portion more in cancer cells than in hyperplasia. Membranous distribution of ezrin also was observed in all the metastatic lesions.10
These results are consistent with a previous cellular based study; the movement of ezrin from the cytoplasm to the membranous portion, such as microvilli, membrane ruffles, cellular extension and foot processes is commonly observed in the highly metastatic cancer cell and is thought to play an important role in the early stages of tumor invasion.40
In addition to contributing to metastatic behavior of cancer cells, ezrin expression is also relevant to the prognosis of cancer. A recent report showed that ezrin expression is related to poor prognosis in the International Federation of Gynecology and Obstetrics (FIGO) stage I endometrioid carcinomas. In this study, they also observed a shift of ezrin expression from the apical membrane to cytoplasmic distribution in carcinomas when compared with normal endometrium. The cytoplasmic distribution of ezrin was also observed in free floating cells detached from papillae and in cells at the tumor-host interface. This ezrin shift seemed to loosen the cell to cell adhesion. The study also noted that strong ezrin expression was correlated with reduced overall survival in univariate survival analysis.13
A similar finding was observed in another recent study. Researchers identified ezrin expression on the basal, lateral an apical aspect of the epithelial cells in normal tissue, but ezrin was patchy and uniformly distributed in the cytoplasm of cancer cells. This may mean that the anchoring or tethering effects of ezrin is decreased in cancer cells, specifically in the apical portion, which may promote movement of cancer cells from their original sites to other sites. All of these findings support our hypothesis regarding ezrin's role in metastasis. Therefore, monitoring the level of ezrin in cancer cells may help predict whether or not the cancer will spread.
There are many reasons to believe that ezrin acts as a scaffolding protein of cellular architecture, as a linker molecule between cell to cell and cell to matrix and as a signal transducer for intracellular and/or intercellular communication, but now this molecule has been focused as a potential determinant of metastasis of cancer cell.
A lot of evidences exist that high levels of ezrin expression strongly suggest that metastatic behavior is increased in variable animal cancer cells involving human cancer cells. Furthermore, high levels of ezrin expression are more observed in high potential metastatic cancer cell than low potential metastatic cells. Its versatile abilities, such as enhancing cellular invasiveness, controlling other tethering molecules and activation of metastasis relating proteins make it be one of the most focused molecules in the area of cancer metastasis.
In addition, it is predisposed to move from the apex to the cytoplasmic and/or membranous portion more in cancer cells than in normal cells. These facts imply that ezrin may play a role in cancer progression.
Since ezrin expression is controlled by estrogen, especially, ezrin has become one of the main focusing molecules related to metastasis of gynecologic cancer. Until now, all the reporting data for the correlation between ezrin and metastasis strongly imply that ezrin may play a pivotal role in distant metastasis and their survival in gynecologic cancer cells. It was already reported that ezrin expression is related to poor prognosis in low stage ovarian and endometrial carcinoma. Given that there are limited reports that evaluated the roles of ezrin in gynecologic cancer, further studies are needed to confirm the precise roles of ezrin in the process of gynecologic cancer metastasis. Nevertheless, it is likely that ezrin levels and its cellular position will begin to be used as a metastatic marker for gynecologic cancer.
Figures and Tables
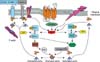
 | Fig. 3A Possible model for activation and function of ezrin. (A) The dormant form of Ezrin with the N-terminal ezrin/radixin/moesin (ERM) associated domain (N-ERMAD) associated with C-ERMAD in cytoplasm. It is transformed into the active form after phosphorylation of specific sites. The N-terminal of activated ezrin can directly bind to C-terminal of transmembrane proteins, and C-ERMAD of ezrin can bind to filamentous actin (F-actin). Ezrin can also act as a linker protein between specific membranous proteins and F-actin via ERM-binding phosphoprotein 50 (EBP50). (B) Activated ezrin can displace guanosine diphosphate (GDP) dissociation inhibitor (GDI) from the Rho-GDI complex, which can stimulate PI4P5 kinase activity. GDP/GTP exchange factor (GEF) catalyzes this reaction. (C) Phosphatidylinositol phosphate (PIP) is changed to phosphatidylinosito (4, 5)-bisphosphate (PIP2) by PI4P5 Kinase. Sequentially PIP2 can change dormant ezrin into its active form. [Modified from "ERM proteins and merlin: integrators at the cell cortex", by Bretscher A, Edwards K and Fehon RG, 2002, Nat Rev Mol Cell Biol, 3, p. 586-99. Copyright 2012 by the RightLink®. Modified with permission]. C: carboxy terminal, N: amino terminal, N-ERMAD: amino-ERM associated domain, C-ERMAD: carboxy-ERM association domain, F-actin: filamentous actin, EBP50: ERM-binding phosphoprotein 50, GDI: guanine nucleotide dissociation inhibitor, PI4P5 Kinase: phosphatidylinositol-4-phosphate 5-kinase, NHE-1, 3: sodium-hydrogen exchanger 1, 3, PDGF-R: platelet derived growth factor receptor, CD44: hyaluronic acid receptor, CD43: leucosialin, ICAM-1, 2: intercellular adhesion molecule-1, 2. |
References
1. Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005. 55:74–108.
2. U.S. Cancer Statistics Working Group. United States Cancer Statistics: 2004 Incidence and Mortality. 2007. Atlanta, U.S: Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute.
3. Buda A, Dell'Anna T, Signorelli M, Mangioni C. Role of ifosfamide in cervical cancer: an overview. Oncology. 2003. 65:Suppl 2. 63–66.
4. Lee CJ, Ariztia EV, Fishman DA. Conventional and proteomic technologies for the detection of early stage malignancies: markers for ovarian cancer. Crit Rev Clin Lab Sci. 2007. 44:87–114.
5. Mehlen P, Puisieux A. Metastasis: a question of life or death. Nat Rev Cancer. 2006. 6:449–458.
6. Bretscher A, Edwards K, Fehon RG. ERM proteins and merlin: integrators at the cell cortex. Nat Rev Mol Cell Biol. 2002. 3:586–599.
7. Curto M, McClatchey AI. Ezrin... a metastatic detERMinant? Cancer Cell. 2004. 5:113–114.
8. Martin TA, Harrison G, Mansel RE, Jiang WG. The role of the CD44/ezrin complex in cancer metastasis. Crit Rev Oncol Hematol. 2003. 46:165–186.
9. Fazioli F, Wong WT, Ullrich SJ, Sakaguchi K, Appella E, Di Fiore PP. The ezrin-like family of tyrosine kinase substrates: receptor-specific pattern of tyrosine phosphorylation and relationship to malignant transformation. Oncogene. 1993. 8:1335–1345.
10. Ohtani K, Sakamoto H, Rutherford T, Chen Z, Kikuchi A, Yamamoto T, et al. Ezrin, a membrane-cytoskeletal linking protein, is highly expressed in atypical endometrial hyperplasia and uterine endometrioid adenocarcinoma. Cancer Lett. 2002. 179:79–86.
11. Bruce B, Khanna G, Ren L, Landberg G, Jirström K, Powell C, et al. Expression of the cytoskeleton linker protein ezrin in human cancers. Clin Exp Metastasis. 2007. 24:69–78.
12. Chen Z, Fadiel A, Feng Y, Ohtani K, Rutherford T, Naftolin F. Ovarian epithelial carcinoma tyrosine phosphorylation, cell proliferation, and ezrin translocation are stimulated by interleukin 1alpha and epidermal growth factor. Cancer. 2001. 92:3068–3075.
13. Köbel M, Langhammer T, Hüttelmaier S, Schmitt WD, Kriese K, Dittmer J, et al. Ezrin expression is related to poor prognosis in FIGO stage I endometrioid carcinomas. Mod Pathol. 2006. 19:581–587.
14. Khanna C, Wan X, Bose S, Cassaday R, Olomu O, Mendoza A, et al. The membrane-cytoskeleton linker ezrin is necessary for osteosarcoma metastasis. Nat Med. 2004. 10:182–186.
15. Bretscher A, Gary R, Berryman M. Soluble ezrin purified from placenta exists as stable monomers and elongated dimers with masked C-terminal ezrin-radixin-moesin association domains. Biochemistry. 1995. 34:16830–16837.
16. Frixione E. Recurring views on the structure and function of the cytoskeleton: a 300-year epic. Cell Motil Cytoskeleton. 2000. 46:73–94.
17. Doherty GJ, McMahon HT. Mediation, modulation, and consequences of membrane-cytoskeleton interactions. Annu Rev Biophys. 2008. 37:65–95.
18. Friedl P, Wolf K. Tumour-cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer. 2003. 3:362–374.
19. Yonemura S, Tsukita S. Direct involvement of ezrin/radixin/moesin (ERM)-binding membrane proteins in the organization of microvilli in collaboration with activated ERM proteins. J Cell Biol. 1999. 145:1497–1509.
20. Lesley J, Hyman R, Kincade PW. CD44 and its interaction with extracellular matrix. Adv Immunol. 1993. 54:271–335.
21. Rodríguez-Rodríguez L, Sancho-Torres I, Leakey P, Gibbon DG, Comerci JT, Ludlow JW, et al. CD44 splice variant expression in clear cell carcinoma of the ovary. Gynecol Oncol. 1998. 71:223–229.
22. Springer TA. Adhesion receptors of the immune system. Nature. 1990. 346:425–434.
23. Arnold JM, Cummings M, Purdie D, Chenevix-Trench G. Reduced expression of intercellular adhesion molecule-1 in ovarian adenocarcinomas. Br J Cancer. 2001. 85:1351–1358.
24. Helander TS, Carpén O, Turunen O, Kovanen PE, Vaheri A, Timonen T. ICAM-2 redistributed by ezrin as a target for killer cells. Nature. 1996. 382:265–268.
25. Reczek D, Berryman M, Bretscher A. Identification of EBP50: a PDZ-containing phosphoprotein that associates with members of the ezrin-radixin-moesin family. J Cell Biol. 1997. 139:169–179.
26. Smith PM, Cowan A, Milgram SL, White BA. Tissue-specific regulation by estrogen of ezrin and ezrin/radixin/moesin-binding protein 50. Endocrine. 2003. 22:119–126.
27. Georgescu MM, Morales FC, Molina JR, Hayashi Y. Roles of NHERF1/EBP50 in cancer. Curr Mol Med. 2008. 8:459–468.
28. Crepaldi T, Gautreau A, Comoglio PM, Louvard D, Arpin M. Ezrin is an effector of hepatocyte growth factor-mediated migration and morphogenesis in epithelial cells. J Cell Biol. 1997. 138:423–434.
29. Kondo T, Takeuchi K, Doi Y, Yonemura S, Nagata S, Tsukita S. ERM (ezrin/radixin/moesin)-based molecular mechanism of microvillar breakdown at an early stage of apoptosis. J Cell Biol. 1997. 139:749–758.
30. Hirao M, Sato N, Kondo T, Yonemura S, Monden M, Sasaki T, et al. Regulation mechanism of ERM (ezrin/radixin/moesin) protein/plasma membrane association: possible involvement of phosphatidylinositol turnover and Rho-dependent signaling pathway. J Cell Biol. 1996. 135:37–51.
31. Barret C, Roy C, Montcourrier P, Mangeat P, Niggli V. Mutagenesis of the phosphatidylinositol 4,5-bisphosphate (PIP(2)) binding site in the NH(2)-terminal domain of ezrin correlates with its altered cellular distribution. J Cell Biol. 2000. 151:1067–1080.
32. Karlsson R, Pedersen ED, Wang Z, Brakebusch C. Rho GTPase function in tumorigenesis. Biochim Biophys Acta. 2009. 1796:91–98.
33. del Peso L, Hernández-Alcoceba R, Embade N, Carnero A, Esteve P, Paje C, et al. Rho proteins induce metastatic properties in vivo. Oncogene. 1997. 15:3047–3057.
34. Tsai MH, Jiang MJ. Rho-kinase-mediated regulation of receptor-agonist-stimulated smooth muscle contraction. Pflugers Arch. 2006. 453:223–232.
35. Takahashi K, Sasaki T, Mammoto A, Takaishi K, Kameyama T, Tsukita S, et al. Direct interaction of the Rho GDP dissociation inhibitor with ezrin/radixin/moesin initiates the activation of the Rho small G protein. J Biol Chem. 1997. 272:23371–23375.
36. Smith PM, Heinrich CA, Pappas S, Peluso JJ, Cowan A, White BA. Reciprocal regulation by estradiol 17-beta of ezrin and cadherin-catenin complexes in pituitary GH3 cells. Endocrine. 2002. 17:219–228.
37. Stemmer-Rachamimov AO, Wiederhold T, Nielsen GP, James M, Pinney-Michalowski D, Roy JE, et al. NHE-RF, a merlin-interacting protein, is primarily expressed in luminal epithelia, proliferative endometrium, and estrogen receptor-positive breast carcinomas. Am J Pathol. 2001. 158:57–62.
38. Ohtani K, Sakamoto H, Rutherford T, Chen Z, Satoh K, Naftolin F. Ezrin, a membrane-cytoskeletal linking protein, is involved in the process of invasion of endometrial cancer cells. Cancer Lett. 1999. 147:31–38.
39. Song J, Fadiel A, Edusa V, Chen Z, So J, Sakamoto H, et al. Estradiol-induced ezrin overexpression in ovarian cancer: a new signaling domain for estrogen. Cancer Lett. 2005. 220:57–65.
40. Weiss L. Metastasis of cancer: a conceptual history from antiquity to the 1990s. Cancer Metastasis Rev. 2000. 19:I–XI. 193–383.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download