Abstract
Objectives
To investigate the cytotoxic effects of lysophosphatidylcholine (lysoPC), an active component of oxidized low-density lipoproteins (LDL), on vascular smooth muscle cells (VSMCs).
Methods
VSMCs were derived from rat aorta. Cell death was assessed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) assay, lactic dehydrogenase (LDH) assay, and DNA fragmentation assay. Apoptosis was quantified by propidium iodide staining and fluorescent activated cell sorting (FACS) analysis, and intracellular free radical production was determined using 2',7'-dichlorofluorescin diacetate (DCF-DA). In addition, the changes in caspases, bcl-2 and bax proteins were evaluated by western blot analysis.
Results
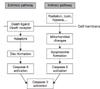
LysoPC over 25 µM induced more than 50% of the cell death at 10 hours on MTT assay with no change in the level of LDH. The DNA ladder pattern showed that cell death induced by lysoPC was caused by apoptosis, which was associated with increased free radical production. Vitamin E, a potent antioxidant and caffeic acid phenylethyl ester (CAPE), an inhibitor of nuclear factor-kappaB (NF-κB), blocked apoptosis. The casepase-3 precursor decreased and the active form of caspase-8 increased. Total bcl-2 and bax proteins did not change with lysoPC treatment, but translocation of bax from cytosole to the mitochondria membrane was observed.
Figures and Tables
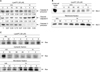
Fig. 1
Cytotoxic effects of lysophosphatidylcholine (lysoPC) on vascular smooth muscle cells (VSMCs). (A) Viability of VSMC treated with lysoPC, (B) cell necrosis induced by lysoPC, (C) lysoPC induced DNA fragmentation.

Fig. 2
Mechanisms for vascular smooth muscle cell (VSMC) apoptosis induced by lysophosphatidylcholine (lysoPC). (A) Involvement of reactive oxygen species and nuclear factor-kappaB (NF-κB), as assessed by propidium iodide (PI) staining, (B) kinetics of reactive oxygen species (ROS) production determined by 2',7'-dichlorofluorescin (DCF) staining. DSF: defined serum-free medium, CAPE: caffeic acid phenylethyl ester. *P < 0.001 vs. control.

References
1. Crisby M, Kallin B, Thyberg J, Zhivotovsky B, Orrenius S, Kostulas V, et al. Cell death in human atherosclerotic plaques involves both oncosis and apoptosis. Atherosclerosis. 1997. 130:17–27.
2. Hsieh CC, Yen MH, Liu HW, Lau YT. Lysophosphatidylcholine induces apoptotic and non-apoptotic death in vascular smooth muscle cells: in comparison with oxidized LDL. Atherosclerosis. 2000. 151:481–491.
3. Steinberg D, Parthasarathy S, Carew TE, Khoo JC, Witztum JL. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med. 1989. 320:915–924.
4. Steinbrecher UP, Parthasarathy S, Leake DS, Witztum JL, Steinberg D. Modification of low density lipoprotein by endothelial cells involves lipid peroxidation and degradation of low density lipoprotein phospholipids. Proc Natl Acad Sci U S A. 1984. 81:3883–3887.
5. Keaney JF Jr, Xu A, Cunningham D, Jackson T, Frei B, Vita JA. Dietary probucol preserves endothelial function in cholesterol-fed rabbits by limiting vascular oxidative stress and superoxide generation. J Clin Invest. 1995. 95:2520–2529.
6. Hamet P, Richard L, Dam TV, Teiger E, Orlov SN, Gaboury L, et al. Apoptosis in target organs of hypertension. Hypertension. 1995. 26:642–648.
7. Isner JM, Kearney M, Bortman S, Passeri J. Apoptosis in human atherosclerosis and restenosis. Circulation. 1995. 91:2703–2711.
8. Kockx MM, De Meyer GR, Muhring J, Jacob W, Bult H, Herman AG. Apoptosis and related proteins in different stages of human atherosclerotic plaques. Circulation. 1998. 97:2307–2315.
9. Owens GK, Loeb A, Gordon D, Thompson MM. Expression of smooth muscle-specific alpha-isoactin in cultured vascular smooth muscle cells: relationship between growth and cytodifferentiation. J Cell Biol. 1986. 102:343–352.
10. Kume N, Cybulsky MI, Gimbrone MA Jr. Lysophosphatidylcholine, a component of atherogenic lipoproteins, induces mononuclear leukocyte adhesion molecules in cultured human and rabbit arterial endothelial cells. J Clin Invest. 1992. 90:1138–1144.
11. Bergmann SR, Ferguson TB Jr, Sobel BE. Effects of amphiphiles on erythrocytes, coronary arteries, and perfused hearts. Am J Physiol. 1981. 240:H229–H237.
12. Ohara Y, Peterson TE, Zheng B, Kuo JF, Harrison DG. Lysophosphatidylcholine increases vascular superoxide anion production via protein kinase C activation. Arterioscler Thromb. 1994. 14:1007–1013.
13. Massaeli H, Pierce GN. Involvement of lipoproteins, free radicals, and calcium in cardiovascular disease processes. Cardiovasc Res. 1995. 29:597–603.
14. Hsu JH, Wu JR, Liou SF, Chen HM, Dai ZK, Chen IJ, et al. Labedipinedilol-a prevents lysophosphatidylcholine-induced vascular smooth muscle cell death through reducing reactive oxygen species production and anti-apoptosis. Atherosclerosis. 2011. 217:379–386.
15. Nishio E, Watanabe Y. Oxysterols induced apoptosis in cultured smooth muscle cells through CPP32 protease activation and bcl-2 protein downregulation. Biochem Biophys Res Commun. 1996. 226:928–934.
16. De Giorgi F, Lartigue L, Bauer MK, Schubert A, Grimm S, Hanson GT, et al. The permeability transition pore signals apoptosis by directing Bax translocation and multimerization. FASEB J. 2002. 16:607–609.
17. Gao CF, Ren S, Zhang L, Nakajima T, Ichinose S, Hara T, et al. Caspase-dependent cytosolic release of cytochrome c and membrane translocation of Bax in p53-induced apoptosis. Exp Cell Res. 2001. 265:145–151.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download