Abstract
Purpose
We analyzed the diagnosis and the treatment outcomes of patients with central low grade osteosarcoma.
Materials and Methods
We retrospectively reviewed 16 patients with central low grade osteosarcoma were treated at out institution between 1994 and 2011.
Results
There were 4 men and 12 women with mean age of 26 years. Eleven patients were correctly diagnosed but 5 patients were misdiagnosed as osteoid osteoma, non ossifying fibroma, aneurysmal bone cyst, desmoplastic fibroma. 15 patients finally received wide margin en bloc excision and one of them treated under neoadjuvant chemotherapy. Final survival status was continuous disease free in 14 and 1 patient died of renal cell cancer. Remaining 1 with multifocal lesions is alive with disease for 7 years only treated radiation therapy on residual tumors. Nine (56%) of 16 tumors showed extraosseous extension of tumor (56%) and 1 of them showed extra-compartmental tumors.
Conclusion
The diagnosis of central low grade osteosarcoma is challenging, however, considering of the clinical suspicion, the typical findings of radiologic and pathologic features, proper diagnosis is needed. This tumor should be treated with wide excision, even after an intralesional excision, to avoid local recurrence or transformation to higher histologic grade.
Go to : 
References
1. Schwab JH, Antonescu CR, Athanasian EA, Boland PJ, Healey JH, Morris CD. A comparison of intramedullary and juxtacortical low-grade osteogenic sarcoma. Clin Orthop Relat Res. 2008; 466:1318–22.

2. Kurt AM, Unni KK, McLeod RA, Pritchard DJ. Low-grade intraosseous osteosarcoma. Cancer. 1990; 65:1418–28.

3. Choong PF, Pritchard DJ, Rock MG, Sim FH, McLeod RA, Unni KK. Low grade central osteogenic sarcoma. A longterm followup of 20 patients. Clin Orthop Relat Res. 1996; 322:198–206.

4. Franceschina MJ, Hankin RC, Irwin RB. Low-grade central osteosarcoma resembling fibrous dysplasia. A report of two cases. Am J Orthop (Belle Mead NJ). 1997; 26:432–40.
5. Kenan S, Ginat DT, Steiner GC. Dedifferentiated high-grade osteosarcoma originating from low-grade central osteosarcoma of the fibula. Skeletal Radiol. 2007; 36:347–51.

6. Muramatsu K, Hashimoto T, Seto S, Gondo T, Ihara K, Taguchi T. Low-grade central osteosarcoma mimicking fibrous dysplasia: a report of two cases. Arch Orthop Trauma Surg. 2008; 128:11–5.

7. Andresen KJ, Sundaram M, Unni KK, Sim FH. Imaging features of low-grade central osteosarcoma of the long bones and pelvis. Skeletal Radiol. 2004; 33:373–9.

8. Bertoni F, Bacchini P, Fabbri N, et al. Osteosarcoma. Low-grade intraosseous-type osteosarcoma, histologically resembling parosteal osteosarcoma, fibrous dysplasia, and desmoplastic fibroma. Cancer. 1993; 71:338–45.

9. Kumar A, Varshney MK, Khan SA, Rastogi S, Safaya R. Low grade central osteosarcoma–a diagnostic dilemma. Joint Bone Spine. 2008; 75:613–5.
10. Hayashi K, Tsuchiya H, Yamamoto N, et al. Diagnosis and treatment of low-grade osteosarcoma: experience with nine cases. Int J Clin Oncol. 2014; 19:731–8.

11. Malhas AM, Sumathi VP, James SL, et al. Low-grade central osteosarcoma: a difficult condition to diagnose. Sarcoma. 2012; 2012:764796.

12. Yoshida A, Ushiku T, Motoi T, et al. Immunohistochemical analysis of MDM2 and CDK4 distinguishes low-grade osteosarcoma from benign mimics. Mod Pathol. 2010; 23:1279–88.

13. Antonescu CR, Huvos AG. Low-grade osteogenic sarcoma arising in medullary and surface osseous locations. Am J Clin Pathol. 2000; 114(Suppl):S90–103.

14. Gisselsson D, Pålsson E, Höglund M, et al. Differentially amplified chromosome 12 sequences in low- and high-grade osteosarcoma. Genes Chromosomes Cancer. 2002; 33:133–40.

15. Tarkkanen M, Böhling T, Gamberi G, et al. Comparative genomic hybridization of low-grade central osteosarcoma. Mod Pathol. 1998; 11:421–6.
Go to : 
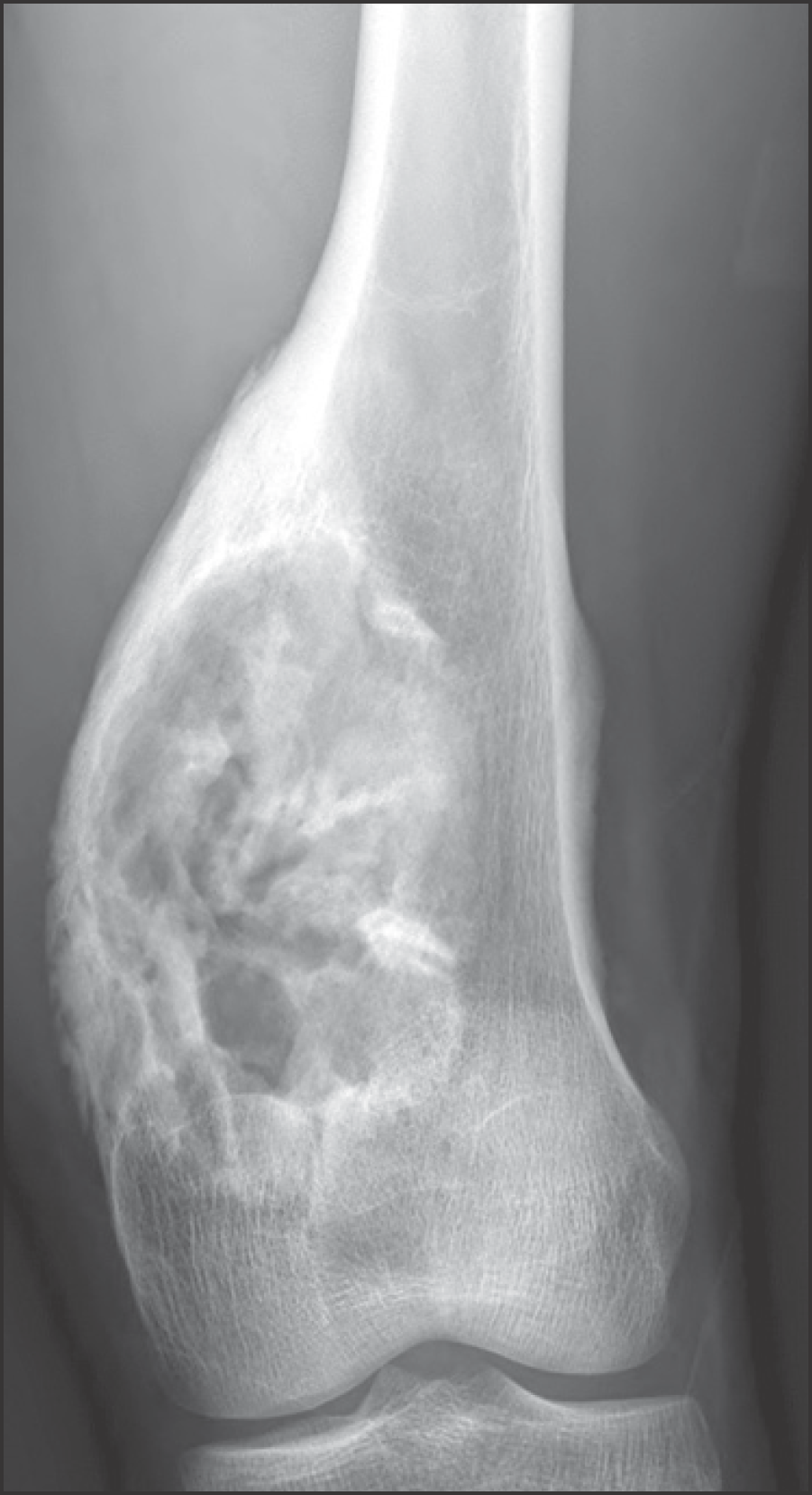 | Figure 1.Plain radiograph of a 21-year-old man showing expansile mixed osteolytic and sclerotic lesion in the distal meta-diaphysis of femur. Radiologic differential diagnosis included osteosarcoma, fibrosarcoma, adamantinoma, and giant osteoblastoma. |
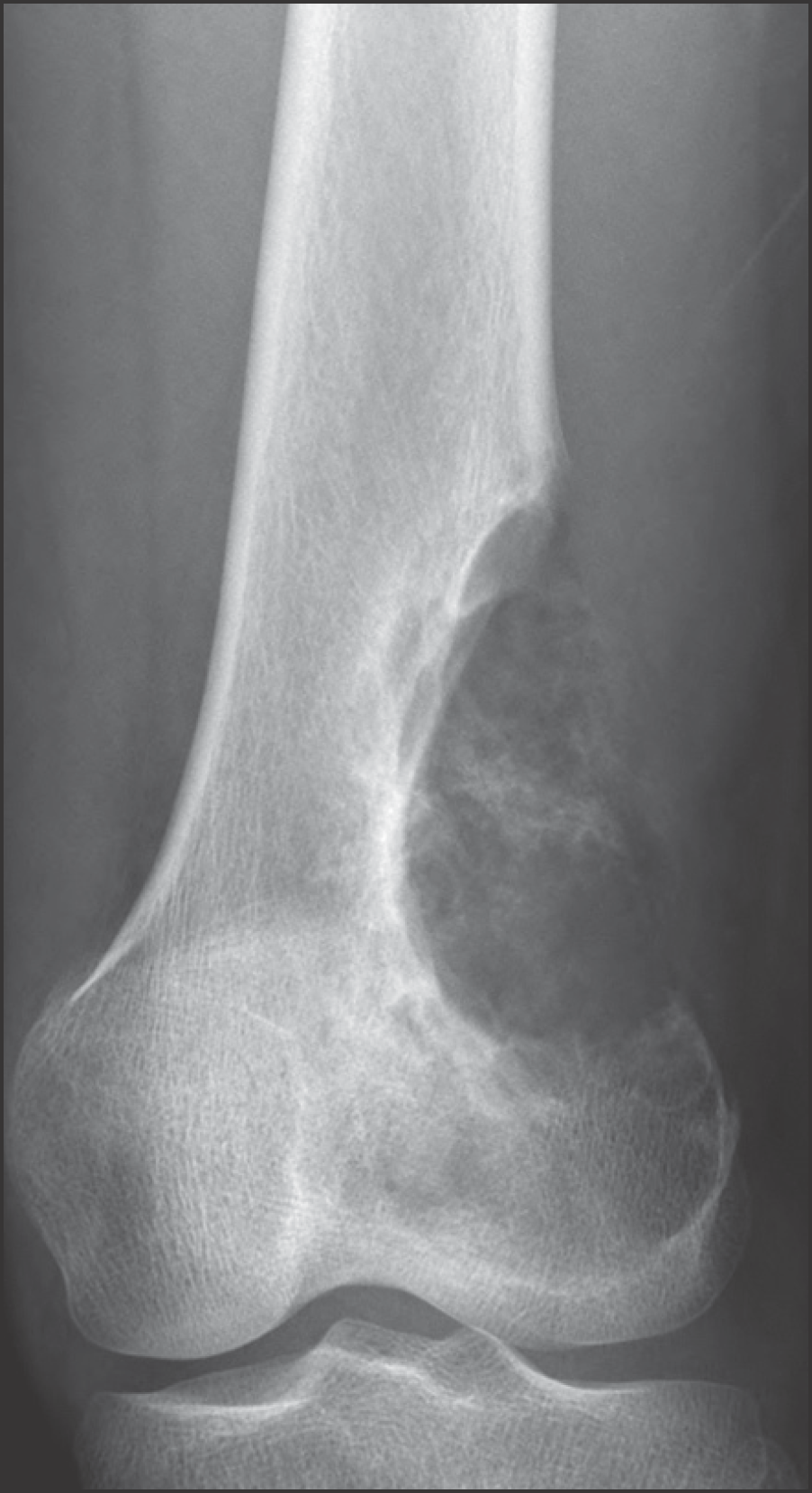
 | Figure 3.Plain radiograph of a 20-year-old man showing predominantly lytic lesion in the distal femur. Radiologic differential diagnosis included osteosarcoma, fibrosarcoma, chondrosarcoma, and giant cell tumor with aneurismal bone cystic change. |
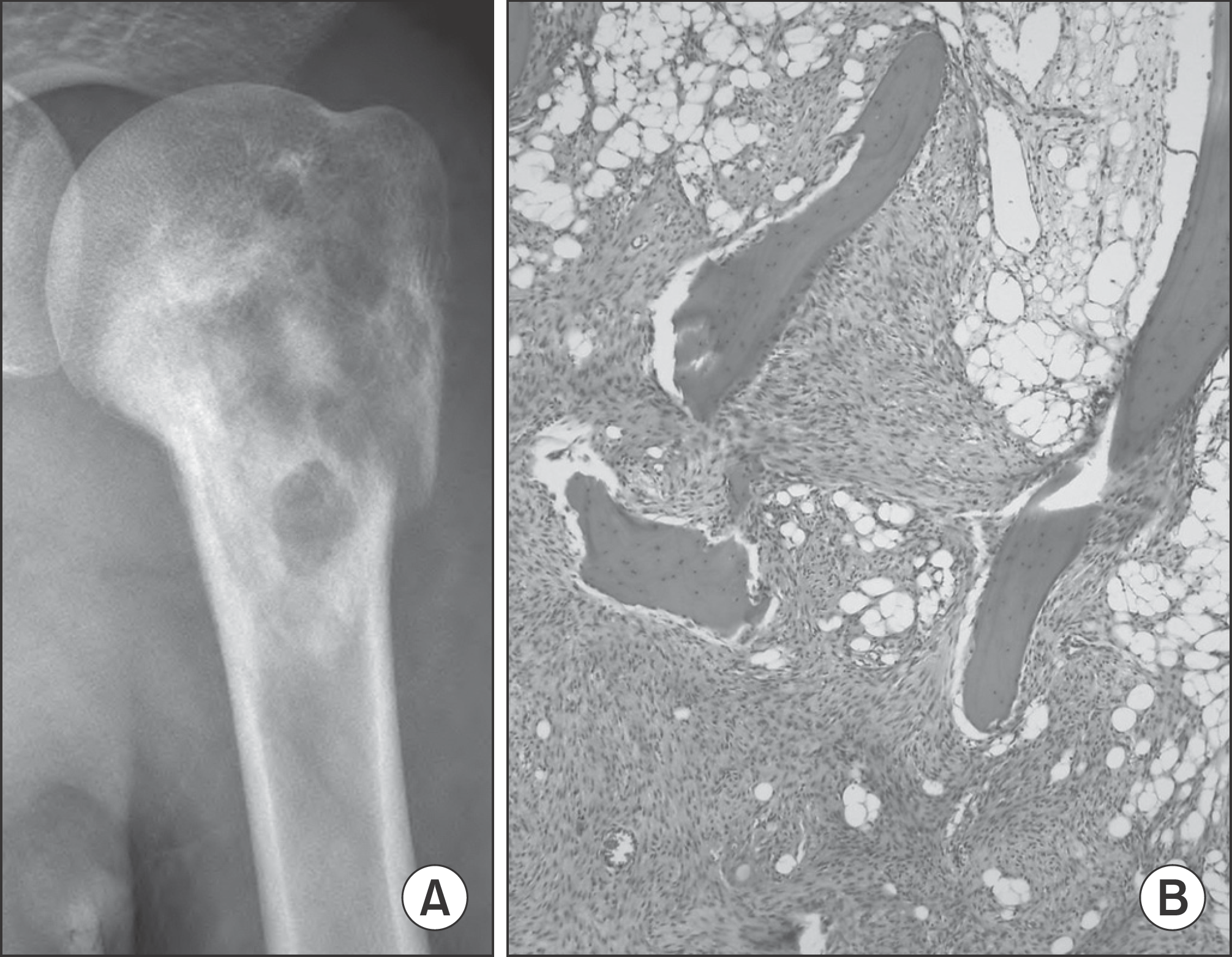
 | Figure 2.(A) Plain radiograph of a 19-year-old girl showing mixed osteolytic and sclerotic lesion in the proximal humerus. (B) The typical histologic appearance of low-grade osteosarcoma, permeation of osseous trabeculae by spindle cell stroma (hematoxylin-eosin, ×100). |
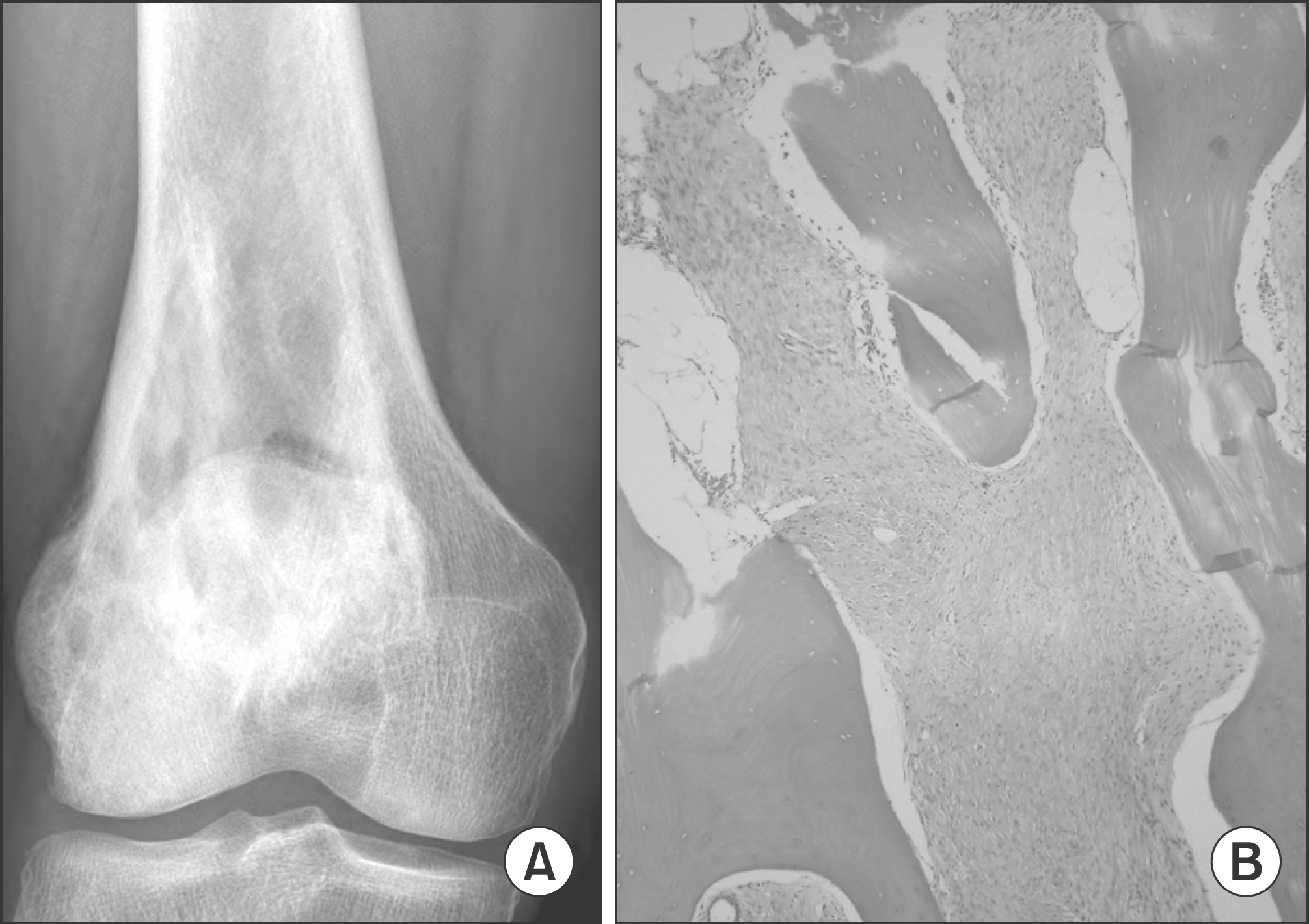 | Figure 4.(A) Plain radiograph of a 54-year-old woman showing sclerotic lesion in the distal femur. (B) Mature bone trabeculae is admixed with permeating spindle cell stroma (×100). |
Table 1.
Patient Characteristics and Radiologic Feature
Table 2.
Surgical Procedure, Immune-Histochemical Analysis, and Clinical Course
Table 3.
Comparison with Previous Studies
| Author | Number of patients | Definitive treatment | Intralesional operation* (%) | Chemotherpy | LR† | Metastasis | Final status |
|---|---|---|---|---|---|---|---|
| Current study | 16 | 13/3 | 3/16 (19%) | 1/16 (6%) | 0% | 0% | NED (15), DOC (1), AWD (1) |
| Choong et al8) | 20 | 12/8 | 12/20 (60%) | – | 14% | 20% | NED (16), DOD (4) |
| Malhas et al12) | 18 | 11/7 | 10/18 (56%) | 7/18 (39%) | 11% | 11% | NED (16), AWD (1), DOD (1) |
| Yoshida et al14) | 9 | 3/6 | 3/9 (33%) | 5/9 (56%) | 14% | 43% | NED (4), AWD (2), DOD (1) |
| Dujardin et al18) | 6 | NA | NA | NA | NA | NA | NA |
| Hayashi et al13) | 5 | NA | 2/5 (40%) | NA | 0% | 0% | NED (5) |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download