Abstract
Purpose
This study was performed to evaluate the efficiency of demineralized bone matrix (DBM, Genesis®) used for bone defect after operative traetment of benign bone tumors by clinical and radiological methods.
Materials and Methods
DBM was used to treat bone defect after operative treatment of benign tumor from February 2012 to May 2013. Total 25 benign bone tumor cases (15 males, and 10 females) with mean age of 30.3 were studied. The diagnoses were solitary bone cyst in 9 cases, non ossifying fibroma in 5, fibrous dysplasia in 5, aneurysmal bone cyst in 3 and enchondroma in 3. In categorization by location of tumor, there were 5 cases of distal femur, 4 of proximal tibia, 3 of proximal femur, 3 of proximal humerus, 3 of phalanx, 2 of distal radius, 2 of hip bone, 2 of calcaneus, and 1 of scapula. Autogenous bone was used with DBM in 6 cases, and only DBM used in 19 cases. Mean periods of follow up were 8.7 months (range: 6 to 14 months). Amount of graft resorption and bone formation was observed with compare of post operation radiograph and the difference was shown by percentage. Resorption level was measured by DBM level which could be observed from simple x-ray, and bone formation level by bone trabecular formation level at impaired site.
Results
Twenty three cases of total 25 cases showed bone union. In the 23 cases, more than 98% DBM resorption was observed after mean 4.3 months, and more than 98% bone formation was observed after mean 6.9 months. Lesser bone defect sizes showed faster bone formation and it was statistically significant (p=0.036). But other comparative studies on other factors such as, sex, age of patients and combination of autogenous bone were no statistically significant differences in graft resorption and bone formation. And there was no significant complication in periods of follow-up.
Go to : 
References
1. Gitelis S, Brebach GT. The treatment of chronic osteomyelitis with a biodegradable antibiotic-impregnated implant. J Orthop Surg (Hong Kong). 2002; 10:53–60.

2. Armstrong JR. Types, sources, and fixation of grafts. Bone-grafting in the treatment of fractures. Baltimore: Williams and Wilkins;1945.
3. Damien CJ, Parsons JR. Bone graft and bone graft substitutes: a review of current technology and applications. J Appl Biomater. 1991; 2:187–208.

4. Greenwald AS, Boden SD, Goldberg VM, Khan Y, Laurencin CT, Rosier RN. American Academy of Orthopaedic Surgeons. The Committee on Biological Implants. Bone-graft substitutes: facts, fictions, and applications. J Bone Joint Surg Am. 2001; 83-A(Suppl 2 Pt 2):98–103.
5. Lind M, Bünger C. Factors stimulating bone formation. Gunzburg R, Szpalski M, Passuti N, editors. The use of bone substitutes in spine surgery. Berlin (Germany): Springer Verlag;18–25.2002.

6. Delloye C, Cnockaert N, Cornu O. Bone substitutes in 2003: an overview. Acta Orthop Belg. 2003; 69:1–8.
7. Hwang C, Bae JY, Koo KH, et al. A Comparative experimental study of allograft andporous hydroxyapatite as bone substitutes. J Korean Orthop Assoc. 2007; 42:545–52.
8. Bucholz RW. Nonallograft osteoconductive bone graft substitutes. Clin Orthop Relat Res. 2002; 395:44–52.

10. Parikh SN, Shital N. Bone graft substitutes in modern orthopedics. Orthopedics. 2002; 25:1301–9.

11. Kelly CM, Wilkins RM, Gitelis S, Hartjen C, Watson JT, Kim PT. The use of a surgical grade calcium sulfate as a bone graft substitute: results of a multicenter trial. Clin Orthop Relat Res. 2001; 382:42–50.
12. Cheng EY, Gebhardt MC. Allograft reconstructions of the shoulder after bone tumor resections. Orthop Clin North Am. 1991; 22:37–48.

13. Clohisy DR, Mankin HJ. Osteoarticular allografts for reconstruction after resection of a musculoskeletal tumor in the proximal end of the tibia. J Bone Joint Surg Am. 1994; 76:549–54.

14. Dick HM, Malinin TI, Mnaymneh WA. Massive allograft implantation following radical resection of high-grade tumors requiring adjuvant chemotherapy treatment. Clin Orthop Relat Res. 1985; 197:88–95.

15. Gebhardt MC, Roth YF, Mankin HJ. Osteoarticular allografts for reconstruction in the proximal part of the humerus after excision of a musculoskeletal tumor. J Bone Joint Surg Am. 1990; 72:334–45.

16. Kim JD, Kang NW, Lee DH, et al. Reconstruction options after surgical resection in Muskuloskeletal Tumors of the Extremity. J Korean Orthop Assoc. 1998; 33:624–36.

17. Mankin HJ, Doppelt S, Tomford W. Clinical experience with allograft implantation. The first ten years. Clin Orthop Relat Res. 1983; 174:69–86.
18. Parrish FF. Allograft replacement of all or part of the end of a long bone following excision of a tumor. J Bone Joint Surg Am. 1973; 55:1–22.

20. Berrey BH Jr, Lord CF, Gebhardt MC, Mankin HJ. Fractures of allografts. Frequency, treatment, and end-results. J Bone Joint Surg Am. 1990; 72:825–33.

21. Bonfiglio M, Jeter WS. Immunological responses to bone. Clin Orthop Relat Res. 1972; 87:19–27.
22. Wang J, Glimcher MJ. Characterization of matrix-induced osteogenesis in rat calvarial bone defects: I. Differences in the cellular response to demineralized bone matrix implanted in calvarial defects and in subcutaneous sites. Calcif Tissue Int. 1999; 65:156–65.

23. Martin GJ Jr, Boden SD, Titus L, Scarborough NL. New formulations of demineralized bone matrix as a more effective graft alternative in experimental posterolateral lumbar spine arthrodesis. Spine (Phila Pa 1976). 1999; 24:637–45.

24. Helm GA, Sheehan JM, Sheehan JP, et al. Utilization of type I collagen gel, demineralized bone matrix, and bone morphogenetic protein-2 to enhance autologous bone lumbar spinal fusion. J Neurosurg. 1997; 86:93–100.

Go to : 
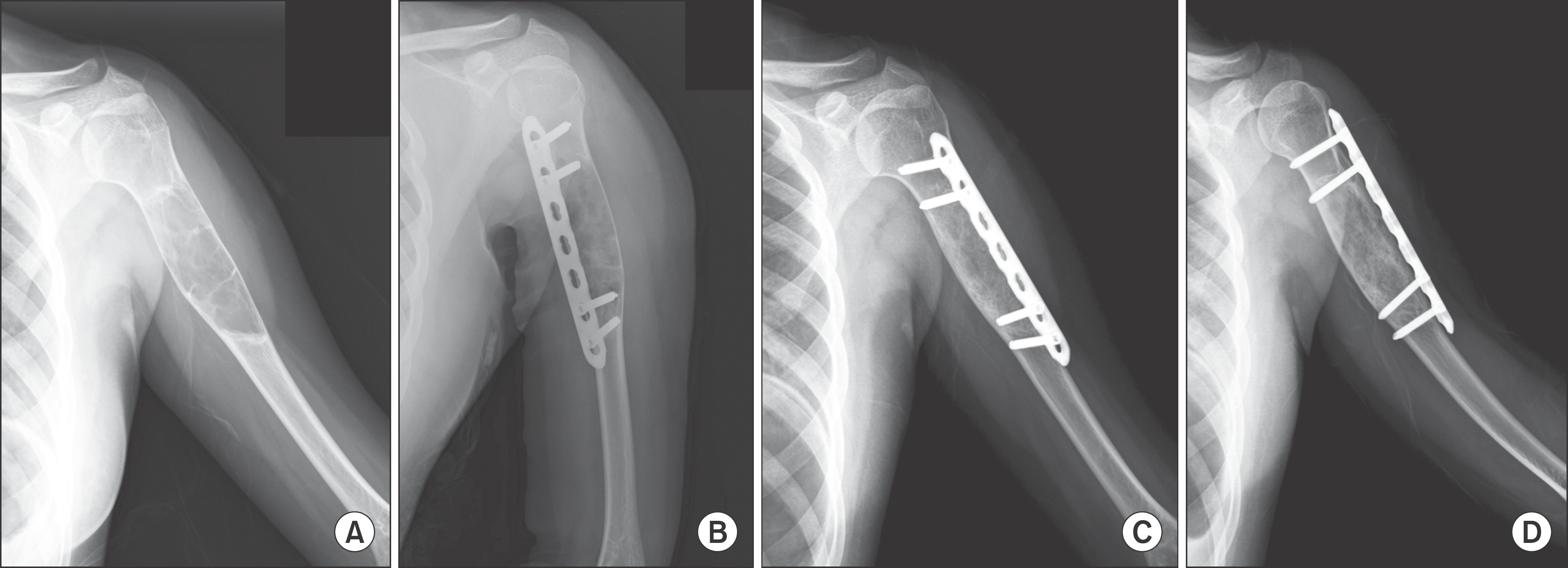 | Figure 2.Solitary bone cyst of Proximal Humerus in a 12-year-old patient. (A) Humerus anteroposterior radiograph shows osteolytic bone lesion on proximal humerus area. (B) Immediate postoperative radiograph shows plate fixation and DBM implantation. (C, D) At 6 months, AP & Lateral radiographs after operation show complete bone union. |
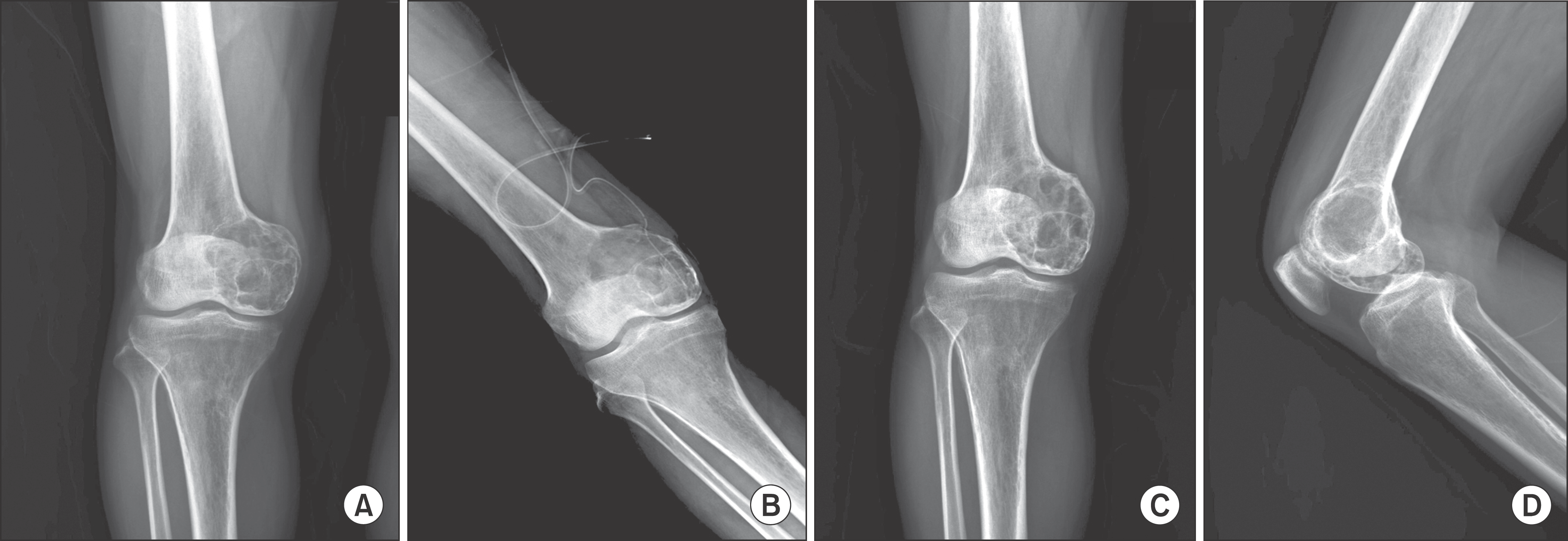 | Figure 3.Aneurysmal bone cyst of Distal Femur in a 51-year-old patient. (A) Initial radiograph shows osteolytic lesion of distal femur. (B) Immediate postoperative radiograph shows Autobone graft and DBM implantation. (C, D) At 13 months, AP & Lateral radiographs after operation show incomplete bone union. |
Table 1.
Types of Bone Tumor
| No. of case | (%) | |
|---|---|---|
| SBC | 9 | 36 |
| NOF | 5 | 20 |
| FD | 5 | 20 |
| ABC | 3 | 12 |
| Enchondroma | 3 | 12 |
| Total | 25 | 100 |
Table 2.
Anatomical Location of the Bone Defect
| Site | No. of case | (%) |
|---|---|---|
| Dist femur | 5 | 20 |
| Prox tibia | 4 | 16 |
| Prox femur | 3 | 12 |
| Humerus | 3 | 12 |
| Hand | 3 | 12 |
| Dist radius | 2 | 8 |
| Pelvis | 2 | 8 |
| Calcaneous | 2 | 8 |
| Scapula | 1 | 4 |
| Total | 25 | 100 |
Table 3.
Demographic Data of the Patients (N=25)
Table 4.
Time for Graft Resorption and Bone Formation




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download