Abstract
Purpose
The purpose of this study is to determine the usefulness of arterial embolization on sacral and pelvic giant cell tumor (GCT).
Materials and Methods
We retrospectively reviewed the medical records of 9 patients who had undergone serial arterial embolization between December 1996 and May 2008. We analyzed the clinical outcomes and therapeutic responsiveness of arterial embolization on sacral and pelvic GCT.
Results
Six of 9 cases showed progression of disease (PD) status, even if 5 cases showed PD status despite of additional treatments including surgery and radiation, implying that serial arterial embolization on sacral and pelvic GCT is not effective. Three of 9 cases showed stable disease (SD) or continuous disease free (CDF) status and we analyzed associated factors with these good responses for embolization by χ2 test. The number of feeding vessels under six (p=0.048) and the number of collateral arterial supply under three (p=0.048) in the first angiogram showed significant relationships with good response for embolization, while remaining tumor staining by contrast after the first embolization and repeated embolization times were not significant.
Go to : 
References
1. Wülling M, Engels C, Jesse N, Werner M, Delling G, Kaiser E. The nature of giant cell tumor of bone. J Cancer Res Clin Oncol. 2001; 127:467–74.

2. Cheng JC, Johnston JO. Giant cell tumor of bone. Prognosis and treatment of pulmonary metastases. Clin Orthop Relat Res. 1997; 338:205–14.

3. Turcotte RE, Sim FH, Unni KK. Giant cell tumor of the sacrum. Clin Orthop Relat Res. 1993; 291:215–21.

4. Campanacci M, Baldini N, Boriani S, Sudanese A. Giant-cell tumor of bone. J Bone Joint Surg Am. 1987; 69:106–14.

5. Kivioja AH, Blomqvist C, Hietaniemi K, et al. Cement is recommended in intralesional surgery of giant cell tumors: a Scandinavian Sarcoma Group study of 294 patients followed for a median time of 5 years. Acta Orthop. 2008; 79:86–93.

6. Balke M, Schremper L, Gebert C, et al. Giant cell tumor of bone: treatment and outcome of 214 cases. J Cancer Res Clin Oncol. 2008; 134:969–78.

7. Wanebo HJ, Koness RJ, Turk PS, Cohen SI. Composite resection of posterior pelvic malignancy. Ann Surg. 1992; 215:685–93. discussion 693–5.

8. Guo W, Sun X, Zang J, Qu H. Intralesional excision versus wide resection for giant cell tumor involving the acetabulum: which is better? Clin Orthop Relat Res. 2012; 470:1213–20.

9. Guo W, Ji T, Tang X, Yang Y. Outcome of conservative surgery for giant cell tumor of the sacrum. Spine (Phila Pa 1976). 2009; 34:1025–31.

10. Leggon RE, Zlotecki R, Reith J, Scarborough MT. Giant cell tumor of the pelvis and sacrum: 17 cases and analysis of the literature. Clin Orthop Relat Res. 2004; 423:196–207.
11. Feigenberg SJ, Marcus Jr RB, Zlotecki RA, Scarborough MT, Berrey BH, Enneking WF. Radiation therapy for giant cell tumors of bone. Clin Orthop Relat Res. 2003; 411:207–16.

12. Caudell JJ, Ballo MT, Zagars GK, et al. Radiotherapy in the management of giant cell tumor of bone. Int J Radiat Oncol Biol Phys. 2003; 57:158–65.

13. Hosalkar HS, Jones KJ, King JJ, Lackman RD. Serial arterial embolization for large sacral giantcell tumors: mid- to longterm results. Spine (Phila Pa 1976). 2007; 32:1107–15.
14. Lackman RD, Khoury LD, Esmail A, Donthineni-Rao R. The treatment of sacral giantcell tumours by serial arterial embolisation. J Bone Joint Surg Br. 2002; 84:873–7.

15. Lin PP, Guzel VB, Moura MF, et al. Long-term follow-up of patients with giant cell tumor of the sacrum treated with selective arterial embolization. Cancer. 2002; 95:1317–25.

16. Martin C, McCarthy EF. Giant cell tumor of the sacrum and spine: series of 23 cases and a review of the literature. Iowa Orthop J. 2010; 30:69–75.
17. Cornelis F, Truchetet ME, Amoretti N, et al. Bisphosphonate therapy for unresectable symptomatic benign bone tumors: A longterm prospective study of tolerance and efficacy. Bone. 2013; 58C:11–6.

18. Chaudhary P, Khadim H, Gajra A, Damron T, Shah C. Bisphosphonate therapy is effective in the treatment of sacral giant cell tumor. Onkologie. 2011; 34:702–4.

Go to : 
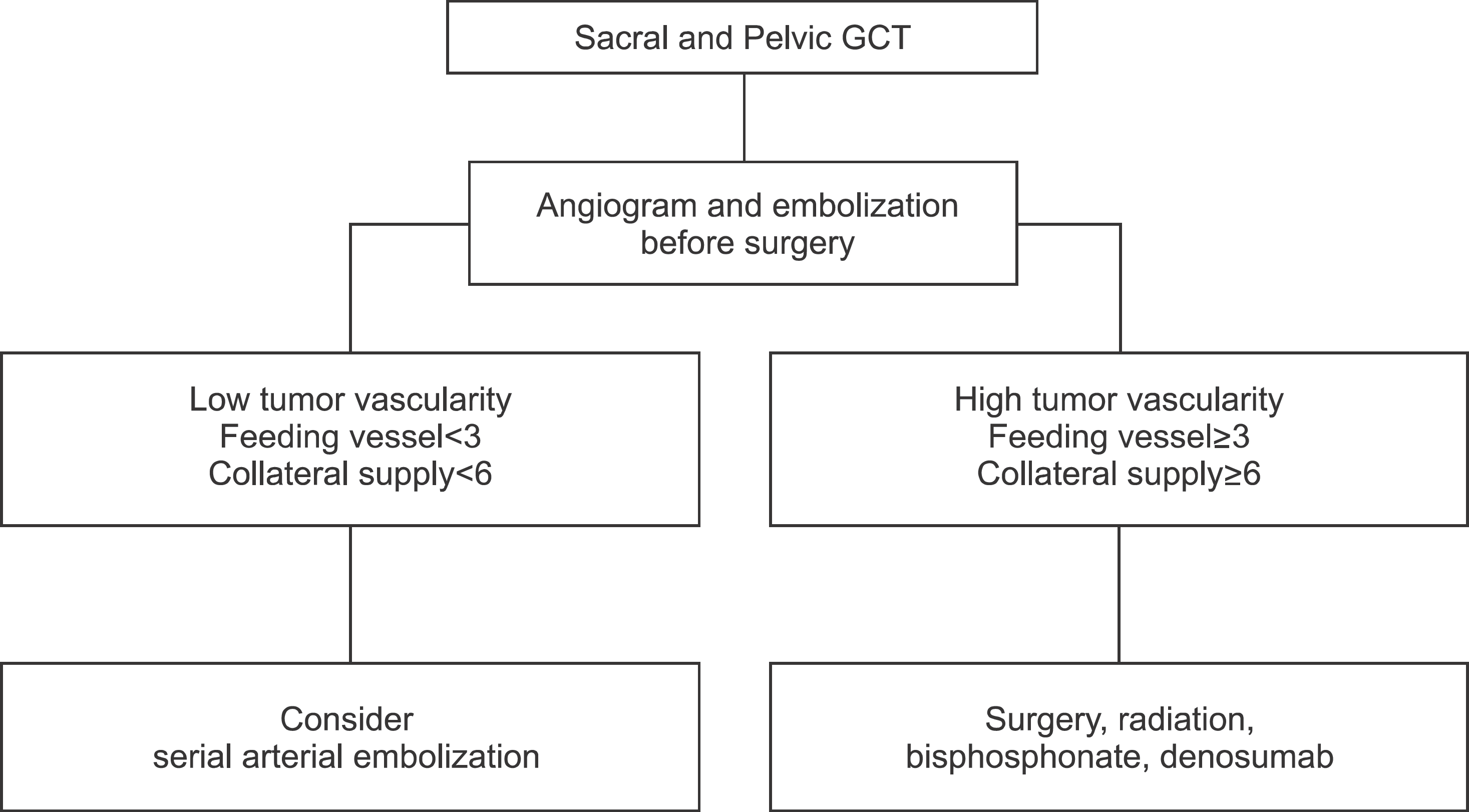 | Figure 1.Algorism for Selecting Treatment Modality on Sacral and Pelvic GCT. GCT, giant cell tumor. |
Table 1.
Treatments and Clinical Outcomes
Table 2.
Tumor Characteristics and Embolization-Responsiveness
Table 3.
χ2 Tests for Evaluating Associations between Clinical Factors and Embolization-Responsiveness
| Clinical factor | Responsive n (%) | Unresponsive n (%) | p* | |
|---|---|---|---|---|
| Sex | Male | 2 (40.0) | 3 (60.0) | 1.000 |
| Female | 1 (25.0) | 3 (75.0) | ||
| Aneurysmal cystic change | Yes | 1 (50.0) | 1 (50.0) | 1.000 |
| No | 2 (28.6) | 5 (71.4) | ||
| ALP elevation | Yes | 1 (50.0) | 1 (50.0) | 1.000 |
| No | 2 (28.6) | 5 (71.4) | ||
| LDH elevation | Yes | 3 (60.0) | 2 (40.0) | 0.196 |
| No | 0 (0.0) | 3 (100.0) | ||
| Main feeding artery | ≥6 | 0 (0.0) | 5 (100.0) | 0.048 |
| <6 | 3 (75.0) | 1 (25.0) | ||
| End artery for feeding artery arousing (Collateral supply) | ≥3 | 0 (0.0) | 5 (100.0) | 0.048 |
| <3 | 3 (75.0) | 1 (25.0) | ||
| Residual tumor staining | Yes | 2 (28.6) | 5 (71.4) | 1.000 |
| No | 1 (50.0) | 1 (50.0) | ||
| Embolization (number of times) | ≥4 | 3 (50.0) | 3 (50.0) | 0.464 |
| <4 | 0 (0.00) | 3 (100.0) |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download