Abstract
Purpose
We investigated the effects of phosphatase and tensin homologue deleted on chromosome 10 gene phosphatase and tensin homologue deleted on chromosome 10 gene (PTEN) expression on the cell proliferation and on the responsiveness of troglitazone in osteosarcoma cells.
Materials and Methods
Western blotting alnalysis was performed to detect the expression of PTEN in U-2OS cells treated with troglitazone. WST (water-soluble tetrazolium) assay was used to evaluate cell proliferation. Flow cytometry was used to determine cell apoptosis. Further, transfection of wild-type PTEN plasmid DNA was used to upregulate PTEN expression.
Results
Troglitazone treatment induced growth inhibition of U2-OS cells in a dose- and time-dependent manner. Troglitazone increased the expression of PTEN in a dose-dependent manner. PTEN upregulation induced by troglitazone treatment resulted in cell growth inhibition and apoptosis in U-2OS cells. PTEN over-expression by plasmid transfection enhanced these effects of troglitazone. Moreover, no changes were observed in the mutant type-PTEN group.
REFERENCES
2. Koeffler HP. Peroxisome proliferator-activated receptor gamma and cancers. Clin Cancer Res. 2003; 9:1–9.
3. Young PW, Buckle DR, Cantello BC, et al. Identification of high-affinity binding sites for the insulin sensitizer rosiglitazone (BRL-49653) in rodent and human adipocytes using a radioiodinated ligand for peroxisomal proliferator-activated receptor gamma. J Pharmacol Exp Ther. 1998; 284:751–9.
4. Kim KY, Kim SS, Cheon HG. Differential anti-proliferative actions of peroxisome proliferator-activated receptor-gamma agonists in MCF-7 breast cancer cells. Biochem Pharmacol. 2006; 72:530–40.
5. Leslie NR, Bennett D, Lindsay YE, Stewart H, Gray A, Downes CP. Redox regulation of PI 3-kinase signalling via inactivation of PTEN. EMBO J. 2003; 22:5501–10.

6. Kim JY, Kim TK, Park JY, Kim HJ, Lee JW. Effects of the peroxisome proliferator-activated receptor ligand troglitazone in osteosarcoma cell lines. J Korean Orthop Assoc. 2005; 40:591–7.

7. Ishiyama M, Miyazono Y, Sasamoto K, Ohkura Y, Ueno K. A highly water-soluble disulfonated tetrazolium salt as a chromogenic indicator for NADH as well as cell viability. Talanta. 1997; 44:1299–305.

8. Ali IU, Schriml LM, Dean M. Mutational spectra of PTEN/MMAC1 gene: a tumor suppressor with lipid phosphatase activity. J Natl Cancer Inst. 1999; 91:1922–32.

9. Zhou XP, Gimm O, Hampel H, Niemann T, Walker MJ, Eng C. Epigenetic PTEN silencing in malignant melanomas without PTEN mutation. Am J Pathol. 2000; 157:1123–8.

10. Moon SH, Lee SH, Kim HS, Kim CH, Chung TW. Phosphatase and Tensin Homologue Deleted on Chromosome 10) in Osteosarcoma. J Korean Orthop Assoc. 2003; 38:39–46.
11. Maehama T, Dixon JE. The tumor suppressor, PTEN/MMAC1 dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-triphosphate. J Biol Chem. 1998; 273:13375–8.
12. Stoll V, Calleja V, Vassaux G, Downward J, Lemoine NR. Dominant negative inhibitors of signalling through the phos-phoinositol 3-kinase pathway for gene therapy of pancreatic cancer. Gut. 2005; 54:109–16.

13. Pedrero JM, Carracedo DG, Pinto CM, et al. Frequent genetic and biochemical alterations of the PI 3-K/AKT/PTEN pathway in head and neck squamous cell carcinoma. Int J Cancer. 2005; 114:242–8.
14. Kreisberg JI, Malik SN, Prihoda TJ, et al. Phosphorylation of Akt (Ser473) is an excellent predictor of poor clinical outcome in prostate cancer. Cancer Res. 2004; 64:5232–6.

15. Cao LQ, Chen XL, Wang Q, et al. Upregulation of PTEN involved in rosiglitazone-induced apoptosis in human hepatocellular carcinoma cells. Acta Pharmacol Sin. 2007; 28:879–87.

16. Yim HW, Jong HS, Kim TY, et al. Cyclooxygenase-2 inhibits novel ginseng metabolite-mediated apoptosis. Cancer Res. 2005; 65:1952–60.

17. Okano H, Shiraki K, Inoue H, et al. 15-deoxy-delta-12-14-PGJ2 regulates apoptosis induction and nuclear factor-kappaB activation via a peroxisome proliferator-activated receptor-gamma-independent mechanism in hepatocellular carcinoma. Lab Invest. 2003; 83:1529–39.
18. Lemberger T, Desvergne B, Wahli W. Peroxisome proliferator-activated receptors: a nuclear receptor signaling pathway in lipid physiology. Annu Rev Cell Dev Biol. 1996; 12:335–63.

19. Jow L, Mukherjee R. The human peroxisome proliferator-activated receptor (PPAR) subtype NUC1 represses the activation of hPPAR alpha and thyroid hormone receptors. J Biol Chem. 1995; 270:3836–40.
20. Caplan AI, Bruder SP. Mesenchymal stem cells: building blocks for molecular medicine in the 21st century. Trends Mol Med. 2001; 7:259–64.

21. Haydon RC, Zhou L, Feng T, Breyer B, et al. Nuclear receptor agonists as potential differentiation therapy agents for human osteosarcoma. Clin Cancer Res. 2002; 8:1288–94.
22. Haydon RC, Luu HH, He TC. Osteosarcoma and osteoblastic differentiation: a new perspective on oncogenesis. Clin Orthop Relat Res. 2007; 454:237–46.
Figure 1.
Growth inhibition effects of troglitazone in the human osteosarcoma U-2OS cell line in a dose (A) and time (B) -dependent manner. Cells (1×105) were treated with various concentrations of troglitazone. Cell viability was determined by the WST assay and was presented as a calculated percentage of viable cells between troglitazone-treated and -untreated control cells. Each points represents the mean SEM of three determinations. †p<0.01 vs. untreated control.
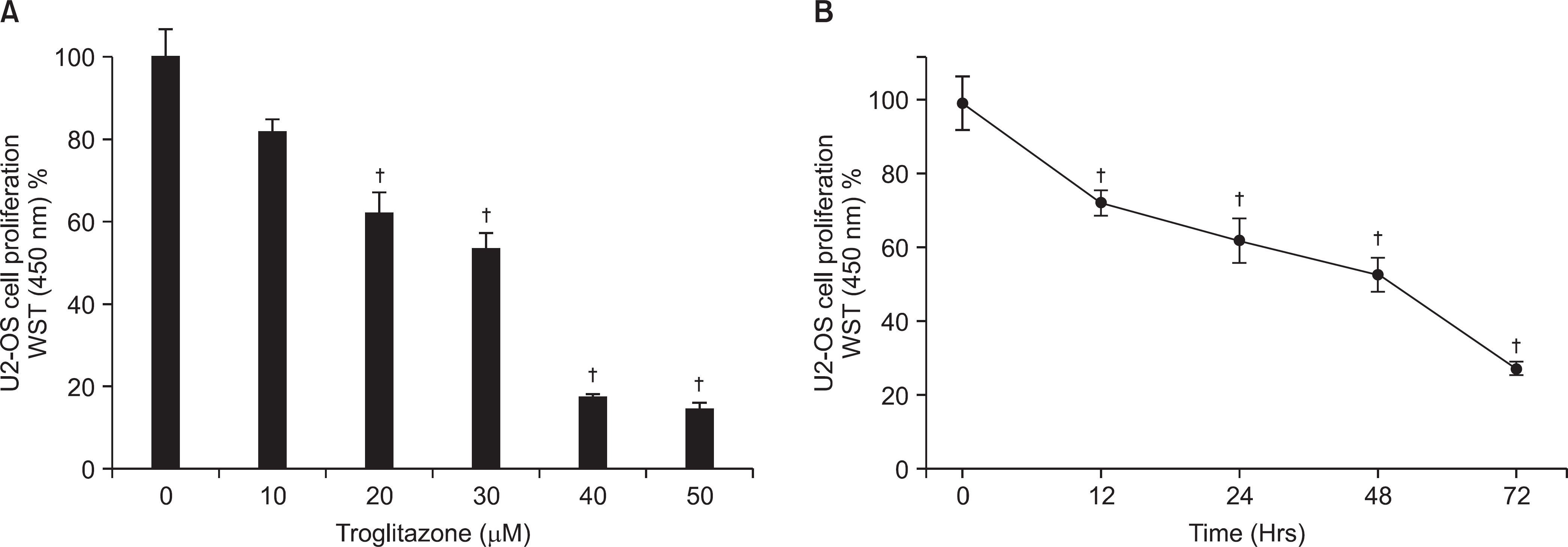
Figure 2.
Effect of troglitazone on the cell survival of osteosarcoma cells by flow cytometry. Histogram patterns of U-2OS cells treated with various concentrations (0, 10, 20, 30, 40, and 50 μM) of troglitazone for 48 hrs by FACS. Cell cycle distribution was analyzed by flow cytometry after coupled staining with Annexin V conjugated to fluorescein isothiocyanate (FITC) and Propidium iodide (PI) as described in Materials and Methods.
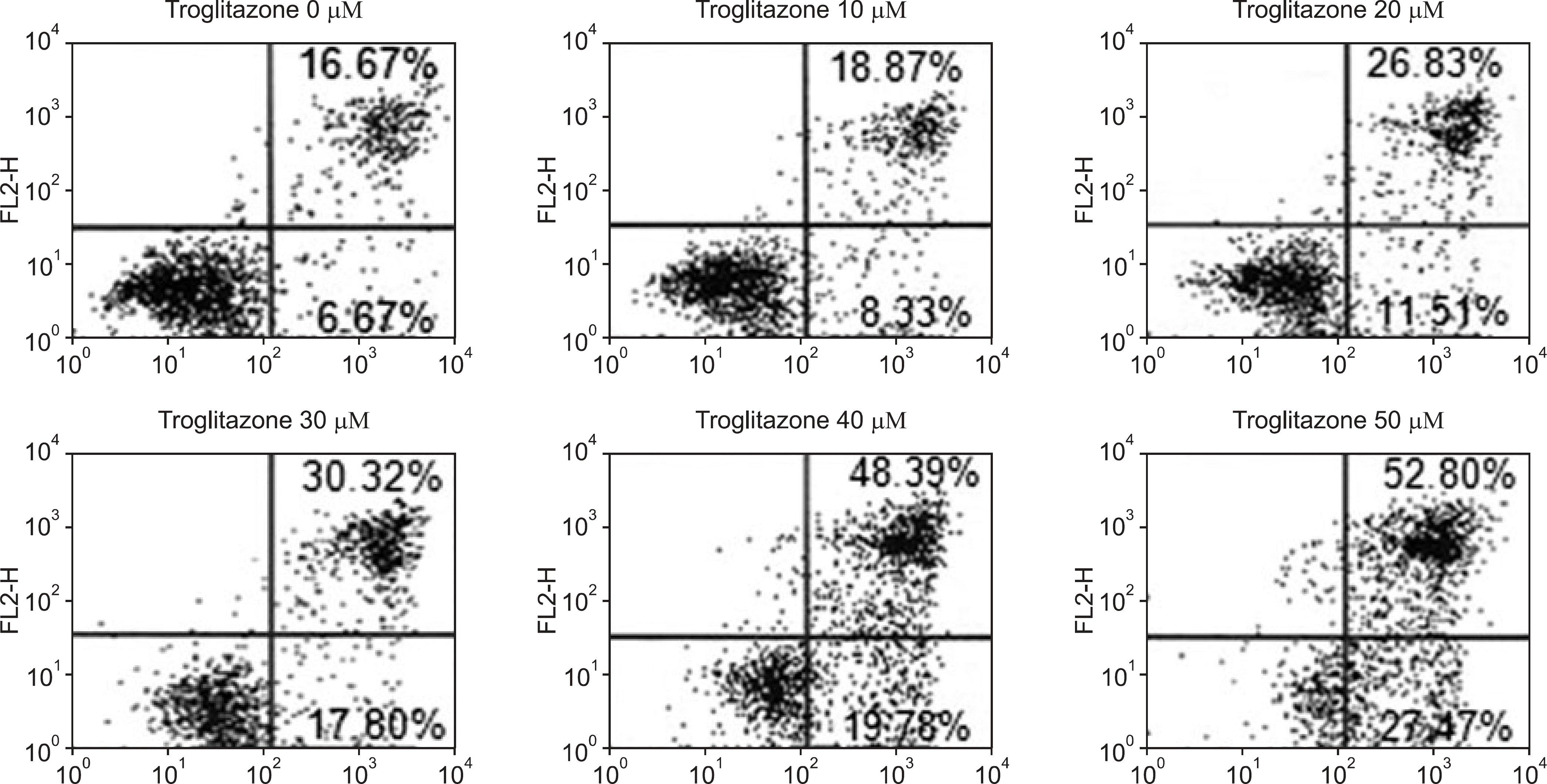
Figure 3.
Dose-dependent effect of troglitazone on the PTEN expression. U2-OS cells (5×106) were treated with 5, 10, 20, and 30 μM of troglitazone for the indicated concentrations. A representative result was presented from among at least three separate experiments yielding similar results. Each points represents the mean SEM of three determinations. ∗p<0.05 vs. untreated control. †p<0.01 vs. untreated control.
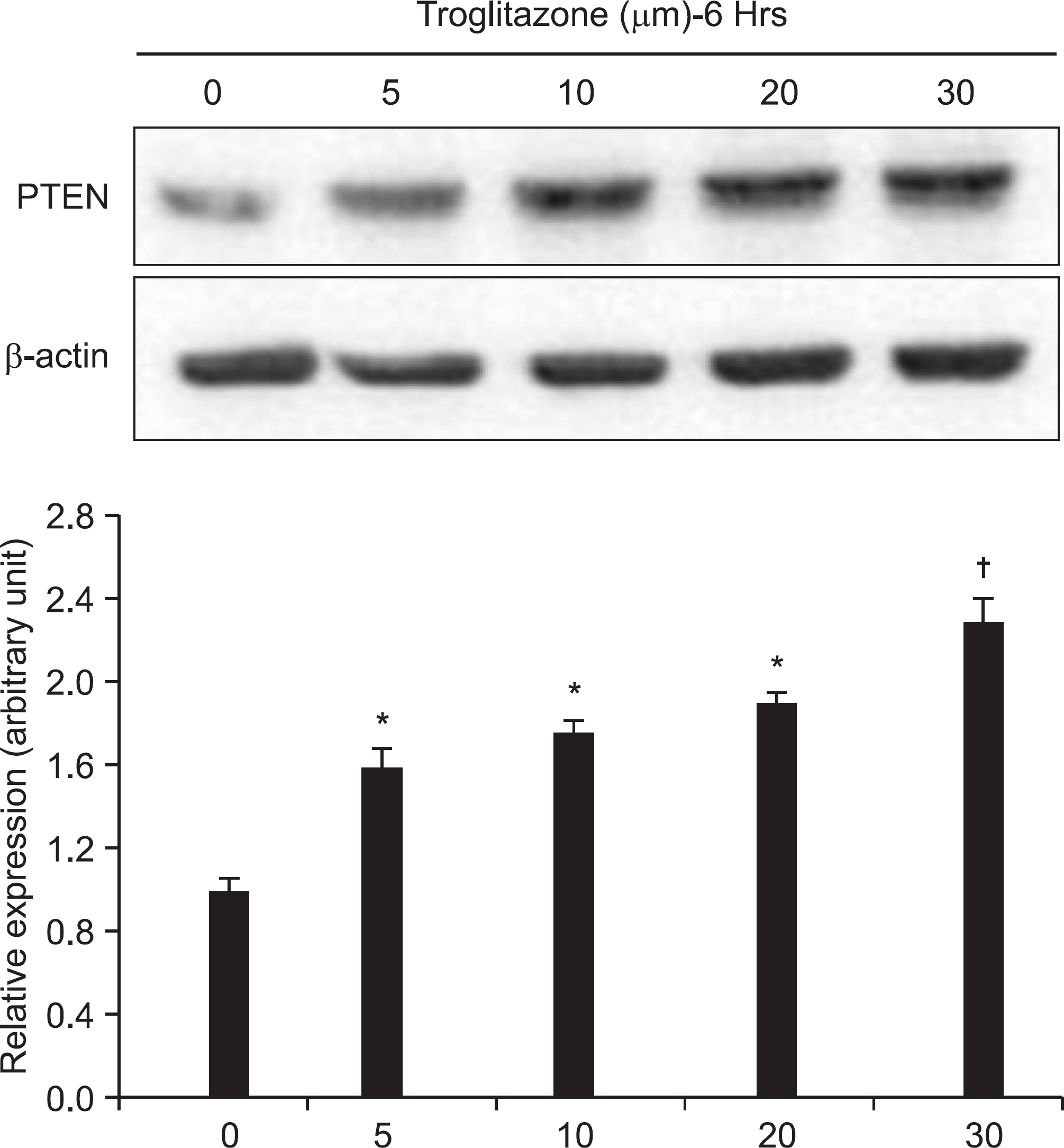
Figure 4.
Establishment of PTEN over-expression osteosarcoma cells using transfection. The PTEN expression in the U-2OS cells declined gradually after being treated with troglitazone for a longer time period (eg 72 hrs), since the activity of plasmid trasfection was decreased with time.
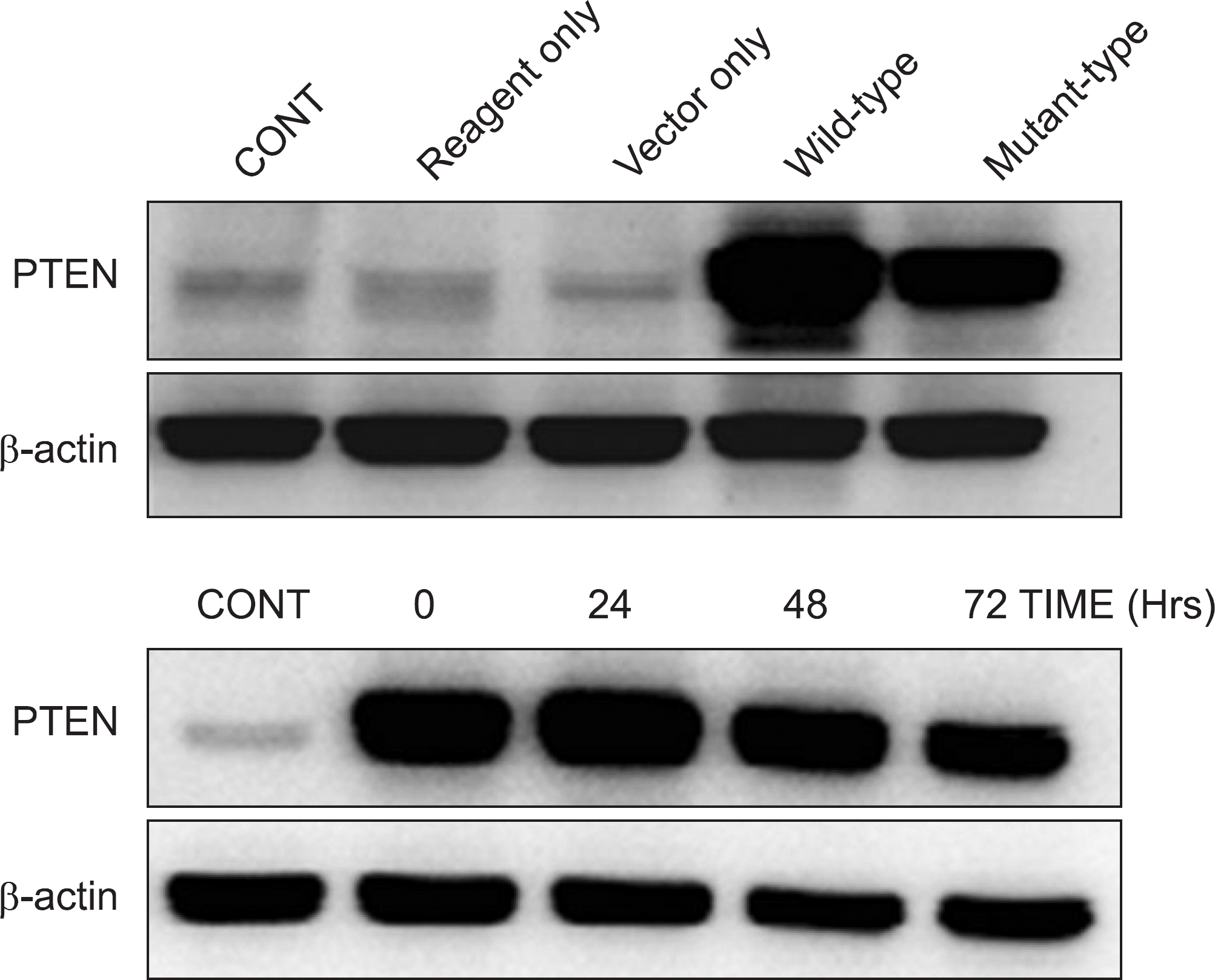
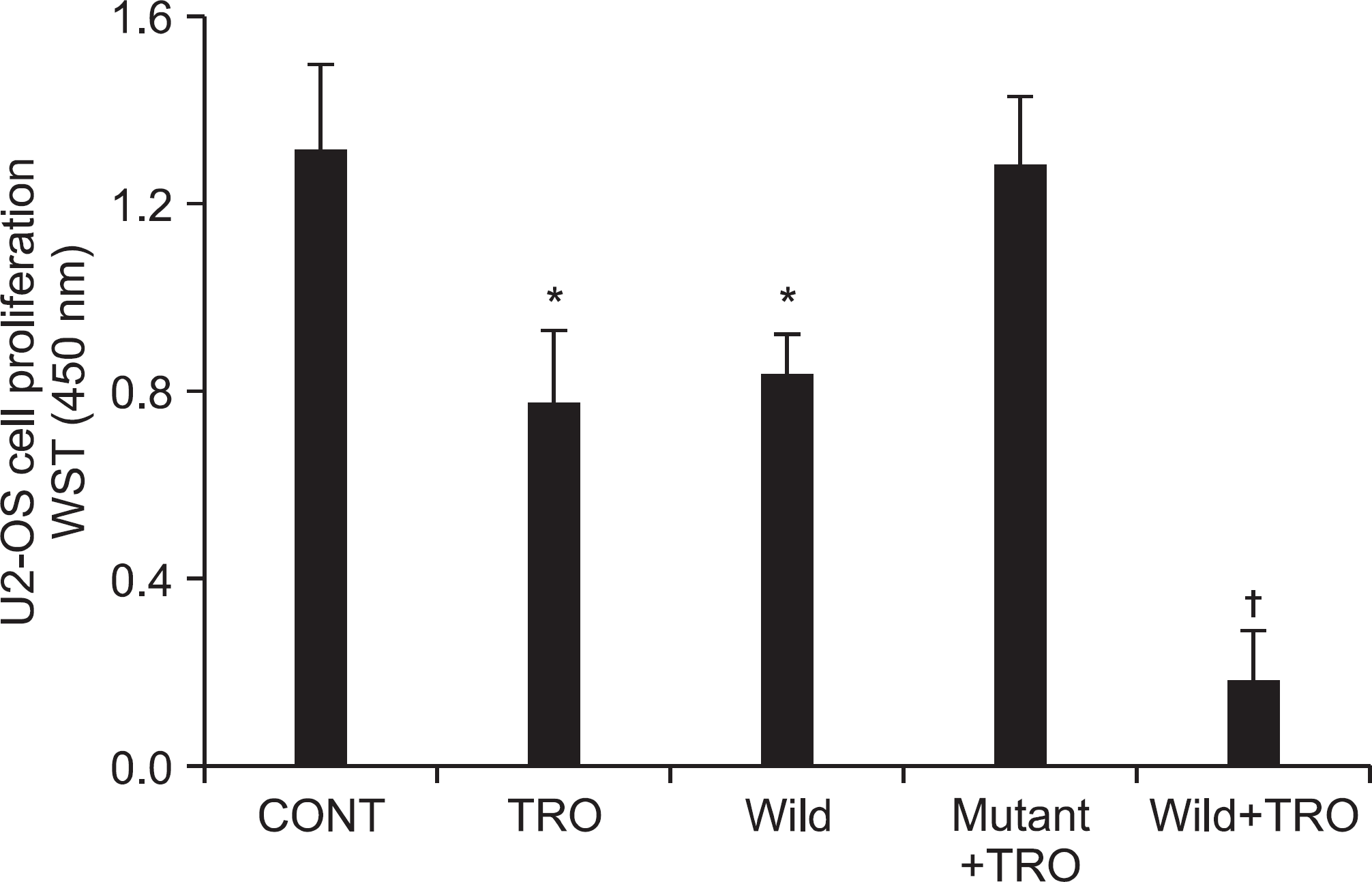
Figure 5.
Transfection of active PTEN in U2-OS cells enhanced the inhibitory effect of troglitazone on cell growth. The effect of troglitazone-decreased cell viability was enhanced in the wild type-PTEN group, whereas no changes were observed in the mutant type-PTEN group. Each points represents the mean SEM of three determinations. ∗p<0.05 vs. untreated control. †p<0.01 vs. untreated control.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download