Abstract
Purpose
This study was performed to evaluate the efficiency of Platelet-rich plasma (PRP) for acceleration of bone healing process on allograft transplantation after curettage in benign bone tumor.
Materials and Methods
From December 2007 to February 2009, twenty-one patients who had benign bone tumor and underwent allograft transplantation after curettage were evaluated. Mean followup period was 14.6 months (range, 12-26 months). We compared with 13 cases of PRP group and 8 cases of non-PRP group in terms of size of lesion, bone resorption, amount of applied PRP and complications. The mean age at surgery was 23.6 years (range, 4-73 years). The most common diagnosis was simple bone cyst (7) followed by enchondroma (4), giant cell tumor (3), undifferentiated benign bone tumor (3) and so on.
Results
The mean size of lesion was 33.5 cm3 (range, 2.3-181.9 cm3) (29.4 cm3 in PRP group and 40.2 cm3 in non-PRP group). The mean volume of injected PRP was 7.4 cc (range, 3-12 cc). Bone union started at 3.0 months (range, 1.5-5.8 months) in PRP group and 5.3 months (range, 4-8 months) in non-PRP group. Three cases for each group were excluded due to recurrence and pathologic fracture. One patient had febrile episode 3 weeks later after surgery which subsided with antibiotics.
REFERENCES
1. Delloye C, Cnockaert N, Cornu O. Bone substituetes in 2003: an overview. Acta Orthop Belg. 2003; 69:1–8.
2. Giannoudis PV, Dinopoulos H, Tsiridis E. Bone substitutes: an update. Injury. 2005; 36(Suppl):20–7.

3. Greenwald AS, Boden SD, Goldberg VM, Khan Y, Laurencin CT, Rosier RN. Bone-graft substitutes: facts, fictions, and applications. J Bone Joint Surg. 2001; 83:98–103.

4. Bauer TW, Smith ST. Bioactive materials in orthopaedic surgery: overview and regulatory considerations. Clin Orthop Relat Res. 2002; 395:11–22.

5. Betz RR. Limitations of autograft and allograft: new synthetic solutions. Orthopedics. 2002; 25(Suppl 5):561–70.

6. Bauer TW, Muschler GF. Bone graft materials. An overview of the basic science. Clin Orthop Relat Res. 2000; 371:10–27.
7. Bostman O, Pihlajamaki H. Clinical biocompatibility of biodegradable orthopaedic implants for internal fixation: a review. Biomaterials. 2000; 21:2615–21.
8. Bouxsein ML, Turek TJ, Blake Ca, et al. Recombinant human bone morphogenetic protein-2 accelerates healing in a rabbit ulnar osteotomy model. J Bone Joint Surg. 2001; 83:219–30.

9. Yeh LC, Unda R, Lee JC. Osteogenic protein-1 differentially regulates the mRNA expression of bone morphogenetic proteins and their receptors in primary cultures of osteoblasts. J Cell Physiol. 2000; 185:87–97.

10. Marx RE, Carlson ER, Eichstaedt RM, Schimmele SR, Strauss JE, Georgeff KR. Platelet-rich plasma: growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Raiol Endod. 1998; 85:638–46.
11. Wrotniak M, Bielecki T, Gazdzik TS. Current opinion about using the platelet-rich gel in orthopaedics and trauma surgery. Orthop Traumatol Rehabil. 2007; 9:227–38.
12. Dallari D, Savarino L, Stagni C, et al. Enhanced tibial osteotomy healing with use of bone grafts supplemented with platelet gel or platelet gel and bone marrow stromal cells. J Bone Joint Surg. 2007; 89:2413–20.

13. Kitoh H, Kitakoji T, Tsuchiya H, et al. Transplantation of marrow-derived mesenchymal stem cells and platelet-rich plasma during distraction osteogenesis - a preliminary result of three cases. Bone. 2004; 35:892–8.
14. Veillette CJ, McKe MD. Growth factors - BMPs, DBMs, and buffy coat products: are there any proven differences amongst them? Injury. 2007; 38(Suppl 1):38–48.

15. Pietrzak WS, Eppley BL. Platelet rich plasma: biology and new technology. J Craniofac Surg. 2005; 16:1043–54.

Figure 1.
Simple bone cyst of the proximal humerus of a 6-year-old patient. (A) The preoperative humerus AP radiograph. (B) PRP was applied after curettage and bone allograft transplantation. (C) Partial resorption of allograft and new trabeculae formation were observed 2.5 months later after operation.
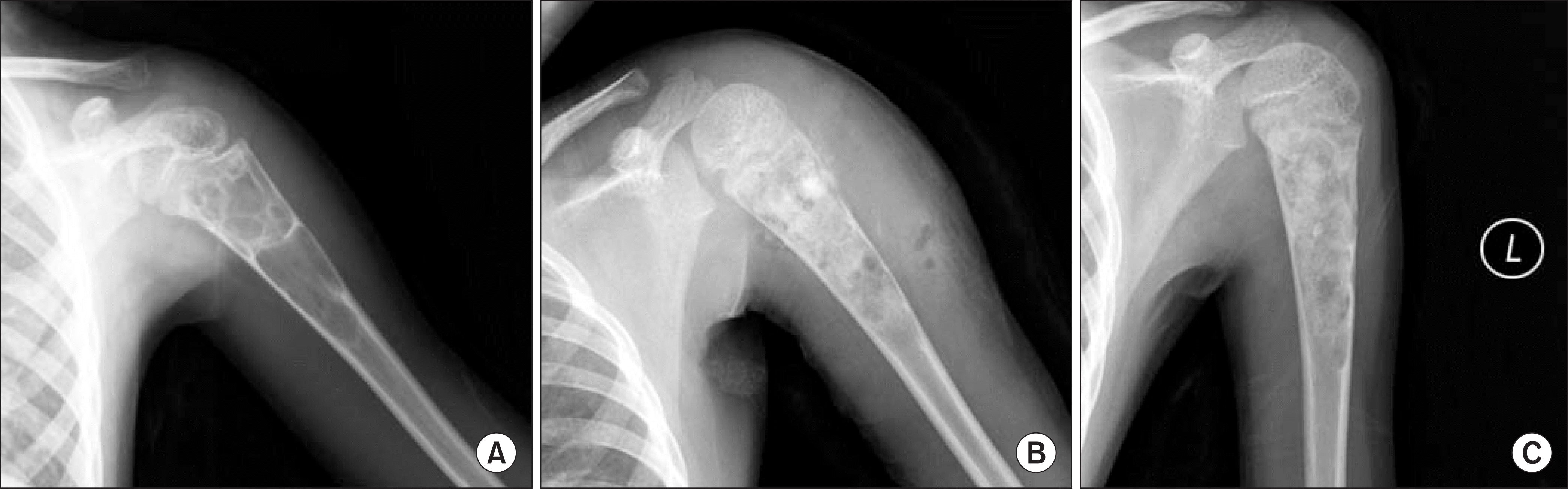
Table 1.
Details of the Patients in PRP Group
Table 2.
Details of the Patients in Non-PRP Group




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download