Abstract
Purpose
With advances in various treatment modalities, limb salvage surgery has been commonly used in osteosarcoma of extremities. An alternative method for skeletal reconstruction is reimplantation of the tumor bearing bone following extracorporeal irradiation (ECI). We report the longterm results of ECI autograft in aspect of the oncological and functional outcomes, and complications.
Materials and Methods
We retrospectively reviewed 31 osteosarcoma patients who underwent reconstruction with ECI between July 1995 and January 2006. There were 24 males and 7 females with a mean age of 24 (7-74 years) and a mean followup of 117 months (17-177 months). Twenty-five cases were reconstructed with ECI autograft, 6 cases with ECI autograft-prosthesis composite. The pathologic subtypes were conventional in 29 cases, periosteal in 1 case, and parosteal in 1 case. The most common location of tumor was distal femur (15 cases) followed by humerus (3), proximal fibula (3) and proximal tibia (3). Musculoskeletal Tumor Society (MSTS) score was used for functional evaluation. The overall survival rate, local recurrence, complications were analyzed.
Results
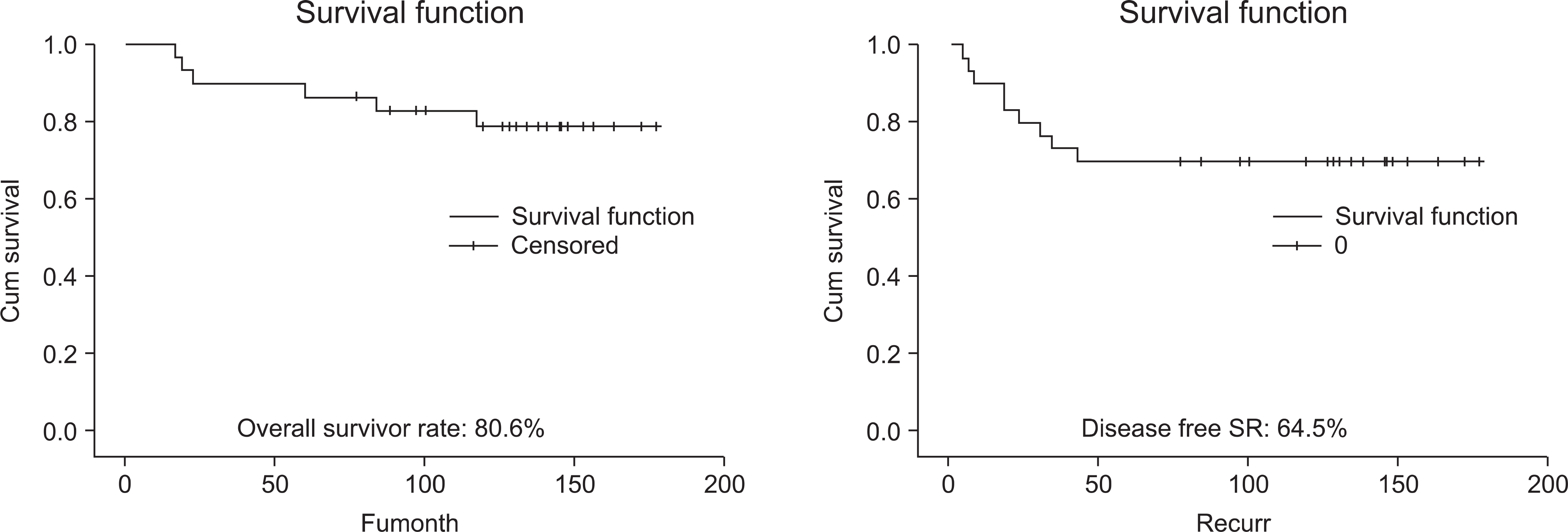
The overall survival rate was 80.6% and the disease-free survival rate was 64.5%. Five patients died of distant metastasis. One patient required above-knee amputation due to local recurrence. All of them, twenty-three complications occurred, which included nonunion in 7 cases, deep infection in 5 cases, joint instability in 4 cases, metal failure in 2 cases, Limb-length discrepancy (LLD) in 2 cases, periprosthetic fracture in 1 case, epiphyseal collapse in 1, local recurrence in 1 case. The mean MSTS functional score was 62.5%.
Conclusion
Extracorporeal irradiated autograft can be achieved relatively good result in aspect of oncological and functional aspect, but is needed to be additional research about occurring many complications. The reconstruction with ECI after intercalary or fragmentary resection is effective reconstruction in aspect of oncological and functional result, complications.
Go to : 
REFERENCES
1. Lee SY, Jeon DG, Lee JS, Oh HO, Jung DH. Survival of Stage IIB Osteosarcoma - Limb-salvage Vs Amputation -. J of Korean Orthop Assoc. 1994; 29:1308–15.
2. Hong A, Steven G, Stalley P, et al. Extracorporeal irradiation for malignant bone tumors. Int J Radiation Oncology Biol Phys. 2001; 50:441–7.

3. Chung SH, Cho Y, Kim JD. Recycling total joint autotransplantation in osteosarcoma around knee joint. J of Korean Bone Joint Tumor. 2007; 13:31–6.
4. Enneking WF, Dunham W, Gebhardt MC, Malawar M, Pritchard DJ. A system for the functional evaluation of reconstructive procedures after surgical treatment of tumors of the musculoskeletal system. Clin Orthop Relat Res. 1993; 286:241–6.

5. Johnston JO. Local resection in primary maliganat bone tumors. Clin Orthop Relat Res. 1980; 153:73–80.
6. Salzer M, Knahr K, Kotz R, Ramach W. Cementless prosthetic implants for limb salvage of malignant tumors of the knee joint: clinical results of three modes of anchorage. Ennecking WF, editor. Limb salvage in musculoskeletal oncology. New York: Churchill Livingstone;1987. p. 26–9.
7. Malawer MM, Chou LB. Prosthetic survival and clinical results with use of large-segment replacements in the treatment of high-grade bone sarcomas. J Bone Joint Surg Am. 1995; 77:1154–65.

8. Dick HM, Malinin TI, Mnaymn WA. Massive allograft implantations following radical resection of high-grade tumors requiring adjuvant chemotherapy treatment. Clin Orthop Relat Res. 1985; 197:88–95.
9. Enneking WF, Mindell ER. Observations on massive retrieved human allografts. J Bone Joint Surg. 1991; 73:1123–42.

10. Jensen KL, Johnston JO. Proximal humeral reconstruction after excision of a primary sarcoma. Clin Orthop Relat Res. 1987; 218:247–58.
11. Kohler P, Kreicberg A. Chondrosarcoma treated by reimplantation of resected bone after autoclaving and supplement with allogenic bone matrix. Clin Orthop Relat Res. 1993; 294:281–4.
12. Manabe J. Experimental studies on pasteurized autogenous bone graft. J Jpn Orthop Assoc. 1993; 67:255–66.
13. Manabe J, Kawaguchi N, Matsumoto S, Kuroda H. Limb salvage and reconstruction by pasteurized autogenous bone graft. J Jpn Orthop Assoc. 1993; 67:21–3.
14. Hurson BJ. Limb sparing surgery for patients with sarcomas [editorial]. J Bone Joint Surg Br. 1995; 77:173–4.
15. Shinobu T, Masayki S, Yoshimoto K, et al. Long-lasting tolerance of articular cartilage after experimental intraoperative radiation in rabbits. Clin Orthop Relat Res. 1992; 275:300–5.
16. Sugimoto M, Takahashi S, Toguchida J, Kotoura Y, Shibamoto Y, Yamamuro T. Changes in bone after high-dose radiation. J Bone Joint Surg. 1991; 73:492–7.
17. Spira E, Brenner HT, Lubin E. Extracorporeal irradiation of malignant bone tumors. Chapchal G, editor. Operative treatment of bone tumors. Stuttgart: Thieme;1970. p. 136–40.
18. Chen WM, Chen TH, Huang CK, Chiang CC, Lo WH. Treatment of malignant bone tumors by extracorporeal irradiated autograft-prosthetic composite arthroplasty. J Bone Joint Surg. 2002; 84:1156–61.
19. Bohm P, Fritz J, Thiede S, Budach W. Reimplantation of extracorporeal irradiated bone segments in musculoskeletal tumor surgery: clinical experience in eight patients and review of the literature. Langenbecks Arch Surg. 2003; 387:355–65.
20. Uyttendaele D, Schryver AD, Claessens H. Limb conservation in primary bone tumors by resection, extracoporeal irradiation and reimplantation. J Bone Joint Surg. 1988; 70:348–53.
21. Takahashi S, Sugimoto M, Kotoura Y, et al. Long standing tolerance of articular cartilage after experimenral intraoperative radiation in rabbits. Clin Orthop Relat Res. 1992; 275:300–5.
22. Sabo D, Bernd L, Ewerbeck V, Eble M, Wannenmacher M, Schulte M. Lokal Behandlung primar maligner Knochen-tumoren. Intraoperative extrakorporale Bestrahlung und Replantation. Unfallchirurg. 1999; 102:580–8.
Go to : 
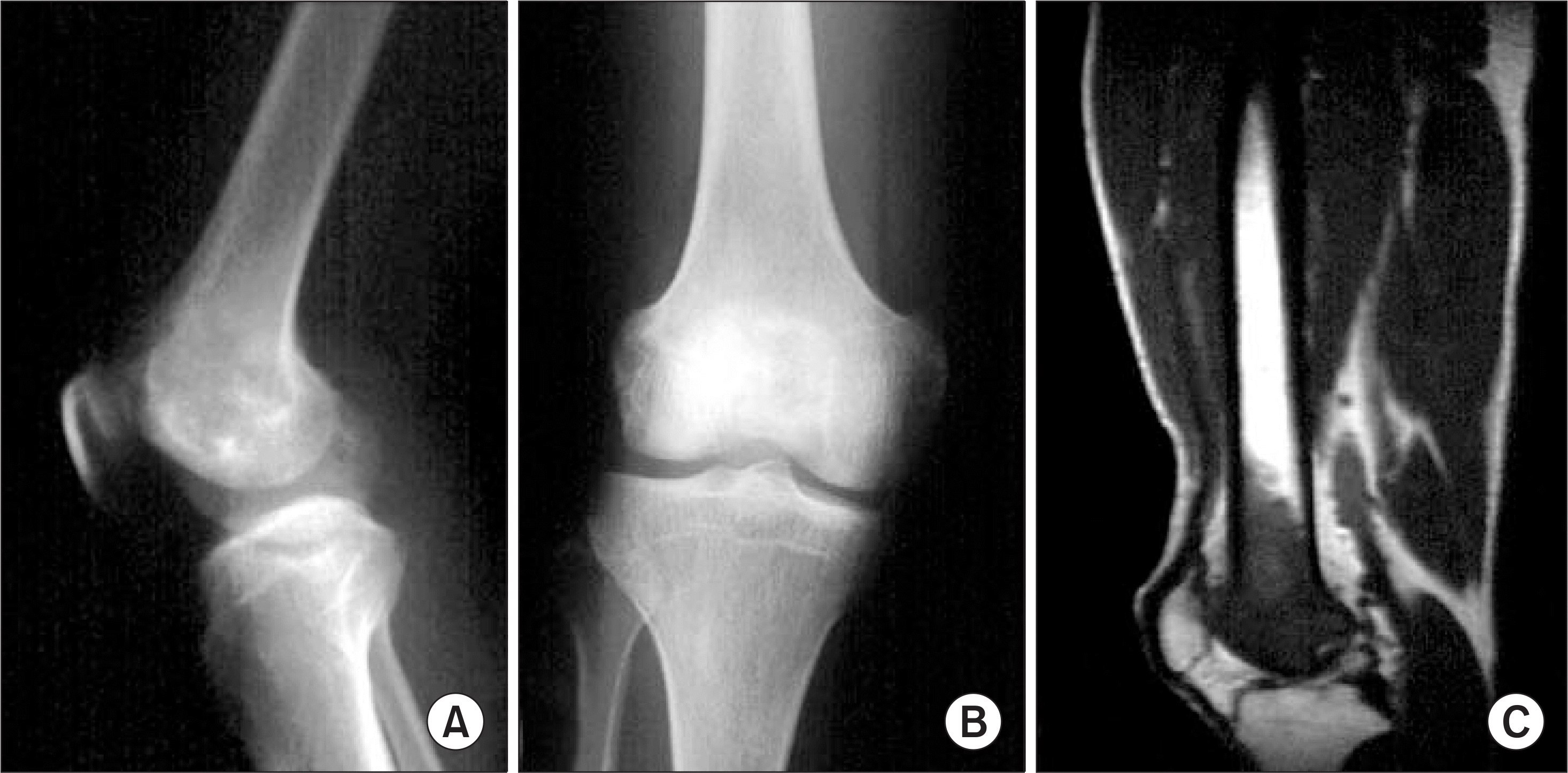 | Figure 2.46 years-old male patient. Preoperative radiograph shows osteosarcoma in the distal femur which invade articular cartilage and knee joint (A, B, C). |
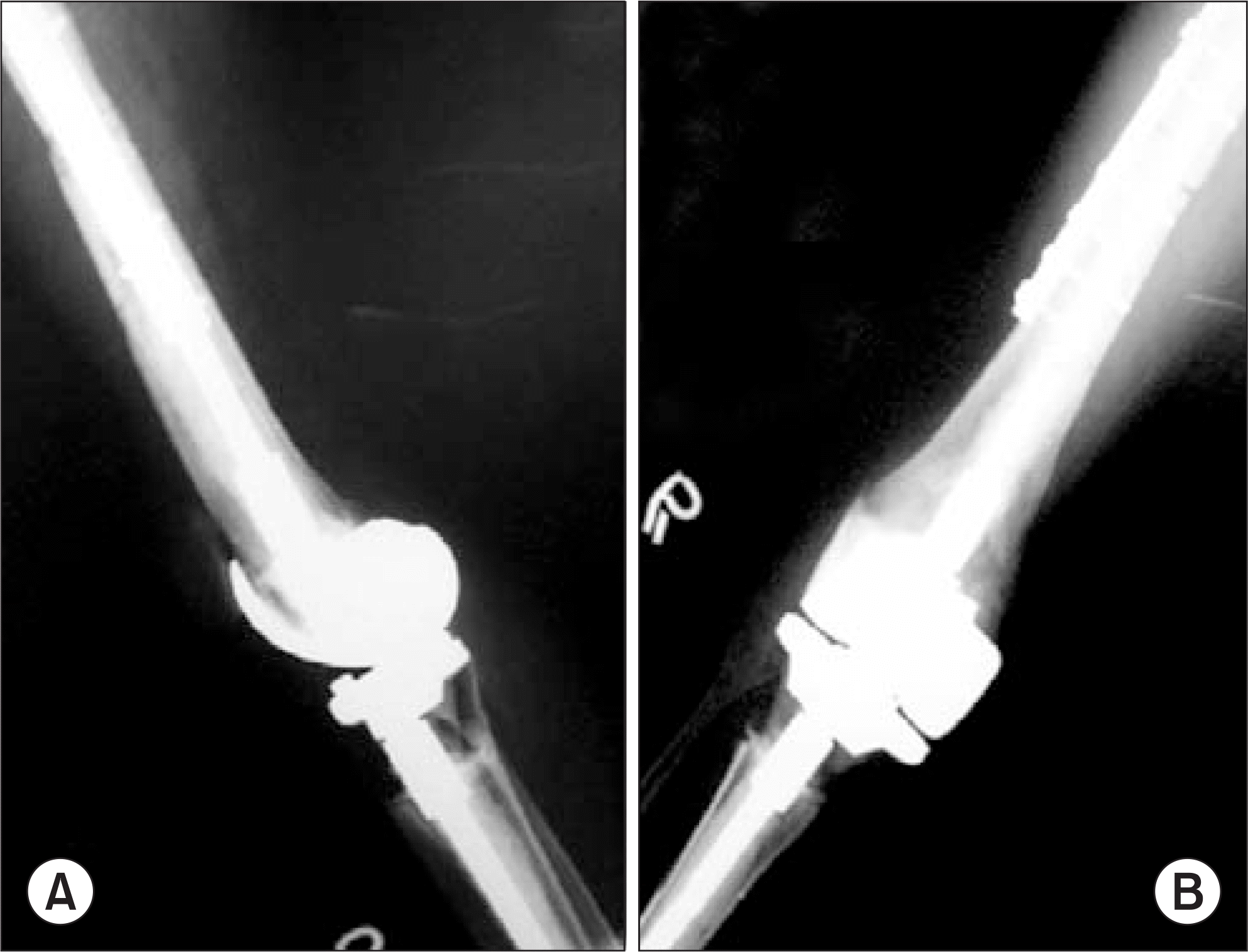
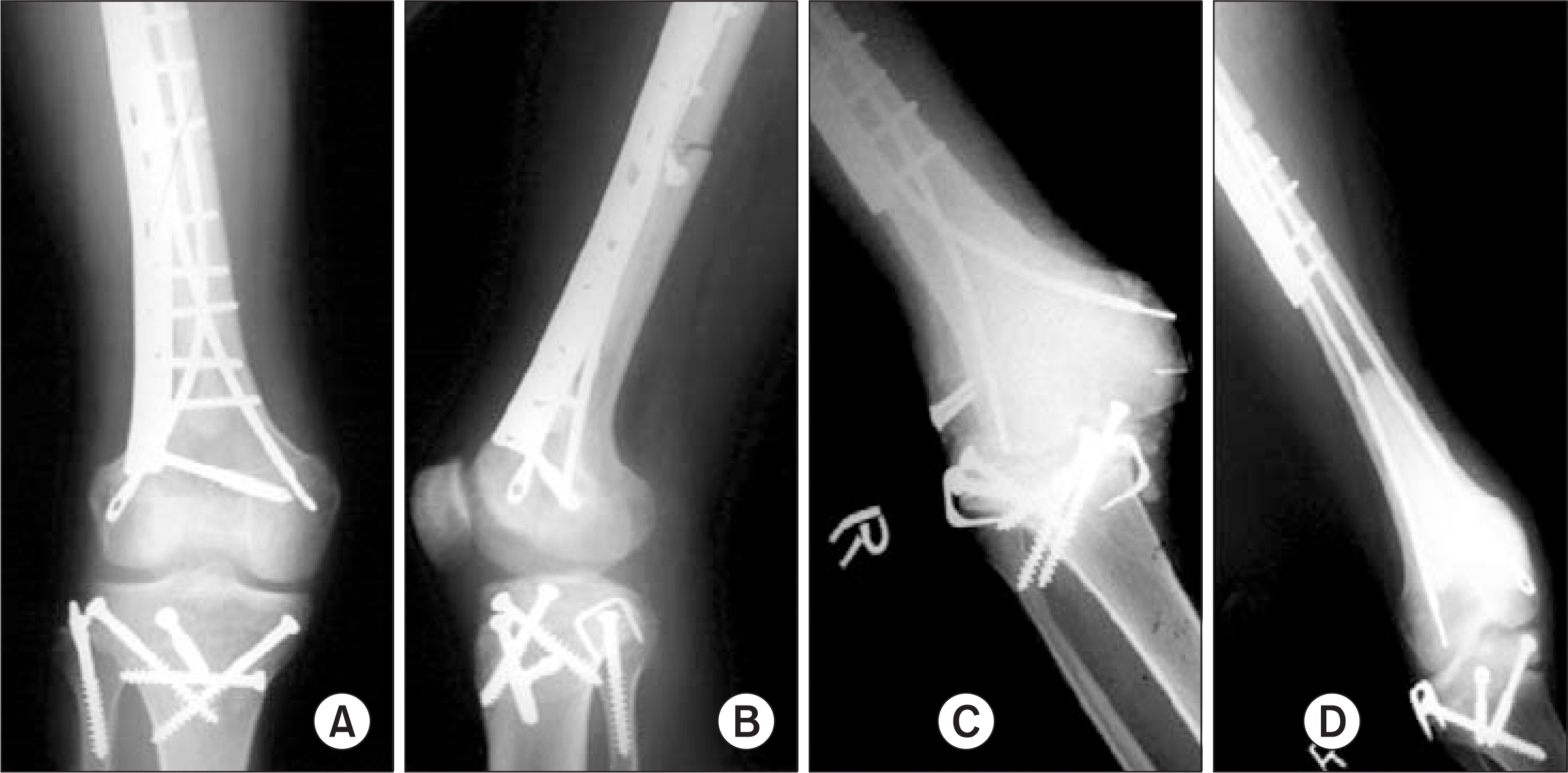 | Figure 3.After total joint type resection, resected bone was reimplanted after extracoporeal irradiation (A, B). About postoperative 6months, joint instability and epiphyseal collapse was occurred (C, D). |
Table 1.
MSTS Functional Results according to Locations of Osteosarcoma
| Total | MSTS score (%) | |
|---|---|---|
| Distal femur | 15 | 61.7 |
| Proximal tibia | 3 | 73 |
| Humerus | 3 | 61.6 |
| Proximal fibula | 3 | 69.7 |
| Proximal femur | 2 | 59.9 |
| Distal tibia | 2 | 76 |
| Pelvis | 2 | 70 |
| Calcaneus | 1 | 70 |
Table 2.
Summarized Data on 31 Patients Reconstructed by ECI for Osteosarcoma




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download