Abstract
Purpose
This study aimed to evaluate the performance of Clinical Research Nurses (CRNs) and the importance of their roles at the Regional Clinical Trial Centers (RCTCs).
Method
A questionnaire focused on the role of CRNs was crafted by a researcher and the content validity was verified by a panel of experts on clinical research. The subjects of this study were 91 CRNs and Clinical Research Coordinators (CRCs), who were Korean registered nurses working at nine RCTCs. 77 subjects yielded valid data were analyzed using descriptive analysis, the Mann-Whitney U test, Spearman's rank order correlation coefficient, and Kruskal-Wallis test.
Results
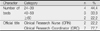
The performance of CRNs and the recognition in the importance of their roles were statistically significant different in age, education, CRN careers, positions, employment status and the phase of clinical trial. The role of direct caregiver was performed most often by CRNs. The role of coordinator of care and research (pre-study) was considered the most important role but performed the least frequent.
References
1. Carpentier WS. Clinical research nurse career ladder. Res Nurse. 1998. 4(5):1–13.
2. Hong JH. South Korea and Japan comparative study of awareness of the clinical trial, researchers. 2009. Seoul: Seoul National University;Unpublished master's thesis.
3. Hwang YS. Job analysis of clinical research nurse in oncology department. 2008. Seoul: Yonsei University;Unpublished master's thesis.
4. Jang IJ. Modanization of Clinical Trial Infrastructure. J Korean Soc Clin Pharmacol Ther. 2006. 14(1):29–32.

5. Jung IS, Kang HS, Kim WO. Current status of educational experience and preferred educational and certification program of clinical research nurses: A questionnaire survey. J Korean Soc Clin Pharmacol Ther. 2005. 13(2):195–207.

6. Kang HS. Developing the educational system for clinical trials (II). 2006. Korea Food & Drug Administration.
7. Kang HS, Jeong IS, Choung SY, Shin SG, Kim WO. Developing the infrastructure for clinical trial in Korea. J Korean Soc Clin Pharmacol Ther. 2004b. 12(2):147–162.

8. Kang HS, Kim WO, Jeong IS, Baik JM. The working conditions and clinical trial practice of research nurses. J Korean Clin Nurs Res. 2004a. 9(2):42–55.
9. Kim JS. Job satisfaction of the clinical research nurse. 2008. Taegu: Kyung-pook University;Unpublished master's thesis.
10. Korea Food & Drug Administration & National Institute of Toxicological Research. Training for clinical trial involved (CRC). 2005.
11. Korea Food & Drug Administration. Korea Good Clinical Practice. 2008.
12. Korea Food & Drug Administration. Bioequivalence Guideline. 2008.
13. Korea National Enterprise for Clinical Trials. http://www.konect.or.kr/.
14. Lee TH. The government's clinical trials industrialization strategy. 1986. 01. 03. Digital Bosa;http://www.digitalbosa.co.kr/.
16. Park MS. The role of RCTC to activate early-phase clinical trials. J Korean Soc Clin Pharmacol Ther - Spring symposium. 2006. 2006, May; 19–24.
17. Rico-Villademoros F, Hernando T, Sanz J, Antonio López-Alonso A, Salamanca O, Camps C, Rosell R. The role of the clinical research coordinator -data manager- in oncology clinical trials. BMC Med Res Methodol. 2004. 4:6. [On-line] http://www.pubmedcentral.nih.gov/.
18. Seoul National University Hospital, Clinical Trials Center. Standard operation procedures. 2008. Seoul: Seoul National University Hospital.
19. Shin JY, Jo SJ, Yim HW, Jung HS, Lee WC, Park YJ, Park YM. Job Satisfaction of Clinical Eesearch Coordinators in a University Hospital. J Korean Soc Clin Pharmacol Ther. 2007. 15(1):57–70.

20. Shin SG, Jang IJ. New drug application process. J Korean Soc Clin Pharmacol Ther. 2001. 9(2):126–137.

21. Song SM. The recognition of KGCP among CRC in an University Hospital located in Seoul. 2008. Seoul: Catholic University;Unpublished master's thesis.




 PDF
PDF ePub
ePub Citation
Citation Print
Print








 XML Download
XML Download