Abstract
BACKGROUND/OBJECTIVES
MATERIALS/METHODS
RESULTS
Figures and Tables
 | Fig. 1Effects of NRM on body weights of mice treated with HFD (45%) and/or DSS.Norm.: normal control mice; HFD: mice treated with a high-fat diet (HFD, 45%); DSS: mice treated with DSS (2%) and a normal diet; HFD+DSS: mice treated with HFD and DSS; HFD+DSS+NL: mice treated with HFD and DSS with 30% NRM in diet; HFD+DSS+NH: mice treated with HFD and DSS with 70% NRM in diet. Results are presented as mean ± SD. Superscript with different letters on 8th week are significantly different (P < 0.05) based on Duncan's multiple range test.
|
 | Fig. 2Effects of NRM on colon length and colon weight-to-length ratio in HFD (45%) and/or DSS-treated mice.Norm.: normal control mice; HFD: mice treated with a high-fat diet (HFD, 45%); DSS: mice treated with DSS (2%) and a normal diet; HFD+DSS: mice treated with HFD and DSS; HFD+DSS+NL: mice treated with HFD and DSS with 30% NRM in diet; HFD+DSS+NH: mice treated with HFD and DSS with 70% NRM in diet. Superscript with different letters on the bars are significantly different (P < 0.05) by Duncan's multiple range test.
|
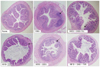 | Fig. 3Histological observations of colon tissue damage in mice treated with NRM, HFD (45%), and/or DSS The arrow (→) indicates specific points of inflammation.Norm.: normal control mice; HFD: treated with a high-fat diet (HFD, 45%); DSS: mice treated with DSS (2%) and a normal diet; HFD+DSS: mice treated with HFD and DSS; HFD+DSS+NL: mice treated with HFD and DSS with 30% NRM in diet; HFD+DSS+NH: mice treated with HFD and DSS with 70% NRM in diet.
|
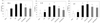 | Fig. 4Effect of NRM on the serum levels of TNF-α, IL-1β, and IL-6 in HFD (45%) and/or DSS-treated mice.Norm.: normal control mice; HFD: mice treated with a high-fat diet (HFD, 45%); DSS: mice treated with DSS (2%) and a normal diet; HFD+DSS: mice treated with HFD and DSS; HFD+DSS+NL: mice treated with HFD and DSS with 30% NRM in diet; HFD+DSS+NH: mice treated with HFD and DSS with 70% NRM in diet. Superscript with different letters on the bars are significantly different (P < 0.05) by Duncan's multiple range test.
|
 | Fig. 5Effect of NRM on the mRNA levels of TNF-α, IL-1β, and IL-6 of HFD (45%) and/or DSS-treated mice.Norm.: normal control mice; HFD: mice treated with a high-fat diet (HFD, 45%); DSS: mice treated with DSS (2%) and a normal diet; HFD+DSS: mice treated with HFD and DSS; HFD+DSS+NL: mice treated with HFD and DSS with 30% NRM in diet; HFD+DSS+NH: mice treated with HFD and DSS with 70% NRM in diet. Band intensities were measured using a densitometer and are expressed as folds of the control (HFD+DSS treated groups). Fold ratio: Gene expression / β-actin × control numerical value (Control fold ratio = 1). Superscript with different letters on the bars are significantly different (P < 0.05) by Duncan's multiple range test.
|
 | Fig. 6Effect of NRM on the mRNA levels of iNOS and COX-2 in the colon tissues of HFD (45%) and/or DSS-treated mice.Norm.: normal control mice; HFD: mice treated with a high-fat diet (HFD, 45%); DSS: mice treated with DSS (2%) and a normal diet; HFD+DSS: mice treated with HFD and DSS; HFD+DSS+NL: mice treated with HFD and DSS with 30% NRM in diet; HFD+DSS+NH: mice treated with HFD and DSS with 70% NRM in diet. Band intensities were measured using a densitometer and are expressed as folds of control (HFD+DSS treated groups). Fold ratio: Gene expression / β-actin × control numerical value (Control fold ratio = 1). Superscript with different letters on the bars are significantly different (P < 0.05) by Duncan's multiple range test.
|
Table 2
Diet Compositions

1) Norm: normal control mice
2) HFD: mice treated with a high-fat diet (HFD, 45%)
3) DSS: mice treated with DSS (2%) and a normal diet
4) HFD+DSS: mice treated with HFD and DSS
5) HFD+DSS+NL: mice treated with HFD and DSS with 30% NRM in diet
6) HFD+DSS+NH: mice treated with HFD and DSS with 70% NRM in diet
Table 3
Effect of NRM on blood chemical indexes (TG, TC, LDL, and HDL) in HFD (45%) and/or DSS-treated mice

1) DSS. Norm.: normal control mice; HFD: mice treated with a high-fat diet (HFD, 45%); DSS: mice treated with DSS (2%) and a normal diet; HFD+DSS: mice treated with HFD and DSS; HFD+DSS+NL: mice treated with HFD and DSS with 30% NRM in diet; HFD+DSS+NH: mice treated with HFD and DSS with 70% NRM in diet.
2) Results are presented as mean ± SD. Superscript with different letters in each column are significantly different (P < 0.05) by Duncan's multiple range test.
Table 4
Effect of NRM on serum insulin, leptin, and adiponectin levels in HFD (45%) and/or DSS-treated mice

1) DSS. Norm.: normal control mice; HFD: mice treated with a high-fat diet (HFD, 45%); DSS: mice treated with DSS (2%) and a normal diet; HFD+DSS: mice treated with HFD and DSS; HFD+DSS+NL: mice treated with HFD and DSS with 30% NRM in diet; HFD+DSS+NH: mice treated with HFD and DSS with 70% NRM in diet.
2) Results are presented as mean ± SD. Superscript with different letters in each column are significantly different (P < 0.05) by Duncan's multiple range test.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download