Abstract
BACKGROUND/OBJECTIVES
Iron deficiency in early life is associated with developmental problems, which may persist until later in life. The question of whether iron repletion after developmental iron deficiency could restore iron homeostasis is not well characterized. In the present study, we investigated the changes of iron transporters after iron depletion during the gestational-neonatal period and iron repletion during the post-weaning period.
MATERIALS/METHODS
Pregnant rats were provided iron-deficient (< 6 ppm Fe) or control (36 ppm Fe) diets from gestational day 2. At weaning, pups from iron-deficient dams were fed either iron-deficient (ID group) or control (IDR group) diets for 4 week. Pups from control dams were continued to be fed with the control diet throughout the study period (CON).
RESULTS
Compared to the CON, ID rats had significantly lower hemoglobin and hematocrits in the blood and significantly lower tissue iron in the liver and spleen. Hepatic hepcidin and BMP6 mRNA levels were also strongly down-regulated in the ID group. Developmental iron deficiency significantly increased iron transporters divalent metal transporter 1 (DMT1) and ferroportin (FPN) in the duodenum, but decreased DMT1 in the liver. Dietary iron repletion restored the levels of hemoglobin and hematocrit to a normal range, but the tissue iron levels and hepatic hepcidin mRNA levels were significantly lower than those in the CON group. Both FPN and DMT1 protein levels in the liver and in the duodenum were not different between the IDR and the CON. By contrast, DMT1 in the spleen was significantly lower in the IDR, compared to the CON. The splenic FPN was also decreased in the IDR more than in the CON, although the difference did not reach statistical significance.
CONCLUSIONS
Our findings demonstrate that iron transporter proteins in the duodenum, liver and spleen are differentially regulated during developmental iron deficiency. Also, post-weaning iron repletion efficiently restores iron transporters in the duodenum and the liver but not in the spleen, which suggests that early-life iron deficiency may cause long term abnormalities in iron recycling from the spleen.
Iron deficiency is by far the most common nutritional deficiency throughout the world. It can affect more than 400 million individuals and is most prevalent in infants, young children, and pregnant women. Iron deficiency has been associated with hematological changes, stunted growth, and decreased cognitive and motor function [123]. Iron is especially crucial during development, as it is required for cell growth and differentiation and proper central nervous system metabolism, such as myelination and neurotransmitter synthesis [456]. The disturbances of iron metabolism during the developmental period, therefore, have significant clinical consequences, which may persist in early childhood and even into adolescence.
Iron metabolism in the body is mainly regulated by modulating iron transports in the duodenum, liver, and reticuloendothelial system, such as macrophages in the spleen [7]. Two major transmembrane proteins that are involved in iron transport are ferroportin (FPN) and divalent metal transporter 1 (DMT1). FPN (also known as IREG1 or SLC40A1) is located at the basolateral membrane of the enterocytes in the brush border of the duodenal epithelium, where iron is transported out of the enterocyte and into portal blood circulation. Also, highly expressed in macrophages, FPN plays a crucial role in the export of iron from macrophages to circulation for reutilization of iron derived from phagocytosed senescent erythrocytes. On the other hand, DMT1 is located in the apical membrane of the enterocytes, where dietary iron is taken up from the lumen of the duodenum. In non-intestinal cells, DMT1 is also involved in intracellular iron transport across the endosomal membrane.
Previous studies have reported that several factors, including dietary iron deficiency, hypoxia, and erythropoietic activity, influence iron absorption [891011], which was mainly associated with changes in duodenal DMT1 [1213]. However, the consequences of dietary iron deficiency very early in life on the regulation iron transporters have not been well characterized. In the present study, therefore, we investigated the effects of iron deficiency during the developmental period on the expression of DMT1 and FPN in key tissues related to iron homeostasis, such as those of the duodenum, liver and spleen. Also, the issue of whether iron repletion during the post-weaning period could reverse changes in iron transporters and restore body iron homeostasis was examined.
Male and female Sprague-Dawley rats (7- to 9-week-old) were purchased from Japan SLC incorporation (SLC, Hamamatsu, Japan). Animals were separately housed in polycarbonate cages at 22 ± 2℃, at 50-60% humidity, and with a 12h light/dark cycle, and all animals were permitted free access to food and deionized distilled water. On the third day of acclimation, one male rat and one female rat were mated overnight. Pregnant rats were randomly assigned to two treatment groups and fed either an iron-sufficient control diet (36 ppm Fe, CON) or iron-deficient diet (< 6 ppm Fe, ID) from gestation day 2 until weaning on the postnatal day 21 (PN21). At weaning, rat pups from iron-deficient dams were either fed the same iron-deficient diet (ID) or switched to the iron-sufficient control diet (IDR) for 4 weeks. Pups from control dams were continued to be fed with the iron-sufficient control diet throughout the study period (CON). At the end of the feeding treatment, rats were anaesthetized with ethyl ether, and blood samples were collected. The liver, spleen, and duodenum were collected, washed with 0.9% saline, and stored -80℃ until further analysis. All procedures were approved by the Institutional Animal Care and Use Committees at Kyung Hee University. (KHUASP(SE)-10-011)
Hematocrit was measured by centrifugation, and hemoglobin concentrations in whole blood were measured by the cyanmethemoglobin method [14]. Serum iron concentration and total iron binding iron capacity were determined according to the standard method of the International Committee for Standardization in Hematology [15]. The percentage of transferrin saturation was calculated from the serum iron and total iron binding capacity.
Liver and spleen tissue nonheme iron concentrations were determined using the method introduced by Torrance and Bothwell [16]. Briefly, for tissue digestion, 0.05g tissues were incubated in an acid solution (10% trichloroacetic acid in concentrated HCl) for 20 hours at 60℃. Digested samples were centrifuged, and supernatants were collected and mixed with a chromogen reagent (0.1% bathophenanthrolin sulphonate and 1% thioglycolic acid). After incubation for 10 min at room temperature, the absorbance at 535 nm was measured. A commercially available iron standard was diluted to various concentrations in deionized water and used for the development of a standard curve.
Total RNA was isolated from liver tissue using the Trizol® reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions [17]. Reverse transcription was carried out with 1µg of total RNA by using PrimeScript™ RT reagent kit (TaKaRa Bio, Otsu, Japan). Real-time PCR was performed using a SYBR® Premix Ex Taq™ II kit (TaKaRa) in a real-time PCR instrument (Bio-Rad, Hercules, CA, USA). Primer sequences for hepcidin were 5'-TGC GCT GCT GAT GCT GAA-3' (forward) and 5'-AGC ATT TAC AGC AGA AGA GGC AT-3' (reverse); for BMP6 (bone morphogenetic protein 6) were 5'-CGC CGC AAT CCT CCT CTT-3' (forward) and 5'-CTT TTG CAT CTC CCG CTT CT-3' (reverse); and for GAPDH were 5'-TCC TGC ACC ACC AAC TGC TTA G-3' (forward) and 5'-TTC TGA GTG GCA GTG ATG GCA-3' (reverse). The following real-time cycling conditions were applied: initial denaturation at 95℃ for 5 minutes and continued with cycles of 95℃ for 15 seconds and 60℃ for 1 minute. Cycle threshold numbers for HAMP and BMP6 were normalized to those of GAPDH, and relative gene expressions in the ID and in the IDR group were presented as a fold difference compared to the CON group.
Western blotting was performed with a whole-cell homogenate of the liver, spleen, and duodenum tissue [18]. Briefly, each tissue was homogenized in a lysis buffer [25 mM Tris-HCl (pH 7.6), 1% NP-40, 1% sodium deoxycholate, 150 mM NaCl, 1% SDS] containing 1 mM phenylmethanesulfonylfluoride and a cocktail of protease inhibitors (Roche, Madison, WI, USA). After centrifugation at 14,000 rpm for 20 min at 4℃, supernatants were collected in a microcentrifuge tube and protein concentrations were assayed by using a BCA protein assay kit (Thermo Fisher Scientific, Rockford, IL, USA). Fifty micrograms of the whole-cell homogenate was separated by 10% SDS/polyacrylamide gel electrophoresis, and transferred to a PVDF membrane (Millipore, Billerica, MA, USA). The membranes were stained with a Ponceau S solution to visualize and confirm equal loading and transfer of proteins among wells. The membrane was then blocked with 5% skim milk in Tris-buffered saline containing 0.01% Tween 20 (TBS-T) for 40 minutes at room temperature and incubated with primary antibodies overnight at 4℃. The primary antibodies used in this study were DMT1 (Alpha Diagnostic International, Owings Mills, USA), FPN, ferritin (Binding site, Birmingham, UK), transferrin receptor (TfR, Invitrogen), and β-actin (Santa Cruz Biotechnology, CA, USA). After being washed 5 times in TBS-T for 5 min each, the membranes were incubated in a secondary anti-rabbit IgG peroxidase-linked antibody (Bio-Rad), anti-mouse IgG peroxidase-linked antibody (Bio-Rad), or anti-sheep IgG peroxidase-linked antibody (Santa Cruz Biotechnology) in TBS-T with 1.25% milk for 40 min at room temperature. The bands were detected using Clarity Western ECL substrate (Bio-Rad), and the image was captured and intensities of each band were quantified by using a chemi-doc imaging system (CLINX science Instrument, Shanghai, China).
Iron deficiency from the gestational period resulted in severe anemia (Table 1); the ID rats had significantly lower hemoglobin and hematocrit than that of the CON rats. Serum iron concentrations and the percentage of transferrin saturation were significantly decreased, and the total iron binding capacity was significantly increased in the ID rats, as compared to the CON rats. Liver iron concentration in the ID rats was only 7.8% of that in the CON rats. Similarly, iron concentrations in the spleen were significantly lower in the ID rats, compared to the CON rats.
Iron repletion from P21 normalized hematology, and no significant difference was found in the hemoglobin, hematocrit, serum iron, total iron binding capacity, and transferrin saturation between the CON and IDR groups (Table 1). Hepatic iron concentrations in the IDR rats were significantly higher compared with the ID rats, but still significantly lower compared with the CON rats. The splenic iron concentrations in the IDR rats were not significantly different from those in the ID rats, and both groups had significantly lower splenic iron concentrations, compared to the CON rats.
In the ID rats, the levels of TfR were significantly increased, and the levels of iron storage protein ferritin were significantly decreased in both liver (Fig. 1A) and spleen (Fig. 1B) tissues, as compared to the CON rats. The TfR and ferritin levels are reciprocally regulated in response to iron status [192021]. Similar to changes in tissue iron concentrations, iron repletion significantly increased the ferritin protein levels in both liver and spleen tissues compared with the ID rats, but did not reach to the levels found in the CON rats (Fig. 1A and Fig. 1B).
The hepatic mRNA level of hepcidin was markedly decreased in the ID rats compared with the CON rats (Fig. 2A). Hepatic hepcidin mRNA of the IDR rats was significantly higher compared with the ID rats but still significantly lower compared with the CON rats. The hepatic BMP6 mRNAs were significantly decreased in the ID rats (0.33 ± 0.04) to about 30% of the levels in the CON rats (Fig. 2B). Hepatic BMP6 mRNA levels were not statistically different between the CON and IDR rats.
Western blot analyses showed that the duodenal levels of FPN protein were significantly higher in the ID rats compared with the CON rats (Fig. 3A). Duodenal DMT1 proteins in the ID rats were also significantly higher by 11-fold compared with the CON rats. On the other hand, iron repletion decreased FPN and DMT1 protein levels in the duodenum, and no significant differences were found in the duodenal FPN and DMT1 protein levels between the CON and IDR rats (P > 0.05).
In the liver, FPN protein levels did not differ among the three groups (Fig. 3B). By contrast, hepatic DMT1 levels were significantly lower in the ID rats compared with the CON rats. The hepatic DMT1 levels in the IDR rats were significantly increased and were not significantly different from those in the CON rats.
In the spleen, DMT1 protein levels in the ID rats were significantly lower compared with the CON rats. The DMT1 protein levels in the spleens of the IDR rats remained significantly lower compared with the CON rats (Fig. 3C). Similarly, the FPN protein levels in the spleens were lower in the IDR rats than those in the CON rats, although the difference did not reach statistical significance.
In this study, iron deficiency from the gestational period resulted in severe depletion of tissue iron storages as well as hematological changes, which were associated with altered levels of iron transporter proteins in the duodenum, liver, and spleen. In the duodenum, both DMT1 and FPN were significantly increased by iron deficiency. As DMT1 and FPN are responsible for the uptake of dietary iron in the duodenum [22], it appears that the expression levels of DMT1 and FPN were up-regulated to increase the absorption rate of the dietary iron in the ID group. On the other hand, in the liver and spleen, only DMT1 was significantly decreased by iron deficiency. In these tissues, the DMT1 is mainly involved in transporting iron across the endosomal membrane into the cytosol after TfR-mediated endocytosis of the transferrin bound iron in the blood [2324]. In our study, the percentage of transferrin saturation in the ID group was only one-third of that in the CON group. This reduction of circulating transferrin-bound iron levels could contribute to the significant down-regulation of DMT1 levels in the liver and the spleen.
In the current study, we demonstrated that iron repletion during the post-weaning period was able to correct the severe iron deficiency observed throughout the fetal period. The four weeks of iron repletion were sufficient enough to restore the levels of serum iron, hematocrit, and hemoglobin to a normal range. Our findings reveal that the basic machinery for erythropoiesis (i.e., the proliferation of blood forming cells, hemoglobin synthesis, etc.) remains intact under developmental iron deficiency, and the body has the capacity to respond quickly to changes in dietary iron levels during the postweaning period. Also, the changes in the blood iron parameters were paralleled with the up-regulation of the iron transporters DMT1 and FPN in the duodenum, where most of dietary iron absorption occurs [13].
Iron is unique in that the amounts of iron absorbed daily (1-2 mg) are only a fraction of the total iron required for the production of hemoglobin and other iron-dependent carriers and enzymes (18-24 mg/day) in the body [25]. Splenic macrophages phagocytize and degrade senescent erythrocytes to recycle iron, and therefore, iron flux from macrophages contributes to the majority of daily body iron needs [26]. It is important to note that, in our study, the reduced levels of DMT1 in the spleen due to gestational iron deficiency were not restored to a normal range despite iron repletion afterward. Given the key role of DMT1 in iron-recycling macrophages to transport iron across phagosomal membranes, the failure of recovering DMT1 in the spleen may result in pathological consequences associated with altered iron homeostasis. Moreover, we observed that FPN, the iron exporter protein, also tended to be decreased in the spleen. Further studies are warranted as to the long-term consequences of developmental iron deficiency to the iron recycling and associated pathological changes in the spleen and other tissues.
It is well known that iron homeostasis at the systemic level is mainly regulated by a peptide hormone called hepcidin. Hepcidin decreases circulating iron concentrations by suppressing iron uptake from enterocytes as well as iron release from macrophages and hepatocytes [27]. It was proposed that hepcidin acts by binding to FPN, inducing its internalization and degradation [2829]. We found that the hepatic expression of hepcidin was strikingly down-regulated by about 1000-fold in the ID group as compared to the CON group. As hepcidin is mainly expressed in the liver, the suppression of hepcidin expression in the liver tissue results in a significant reduction of serum hepcidin [30]. When iron is replete in the diet, hepcidin mRNA levels were significantly higher in the IDR group than the ID group, but still significantly lower than the CON group. Considering the fact that serum iron concentrations were not significantly different between the IDR and the CON groups, our results suggest that the degree of hepcidin expression in the liver is more responsive to tissue iron levels rather than circulating iron levels.
We also showed that hepatic BMP6 mRNA levels were significantly down-regulated in the ID group as compared to the CON, indicating that BMP6 mRNA levels correlate positively with hepcidin levels under iron deficiency. BMP6 acts as an upstream regulator for the hepcidin gene transcription, as the hepcidin gene contains BMP responsive elements in the promoter region [313233]. Consequently, BMP6 is a key positive regulator of hepcidin production, and BMP6 administration has been shown to increase hepcidin mRNA levels and reduce serum iron concentrations in mice [34]. Interestingly, when iron is supplemented in the diet (IDR group), the mean level of BMP6 mRNA was significantly increased to a level that is comparable to that of the CON group despite the fact that hepcidin mRNA was still significantly low. This observation indicates that factors other than BMP6 mediate the regulation of hepcidin expression during iron repletion. While the BMP6 signaling pathway represents one of the major regulatory mechanisms, the up-regulation of hepcidin expression by BMP6 can be negatively modulated by soluble hemojuvelin (sHjv). Expressed highly in muscle tissue, sHJV acts as an antagonist of hepcidin expression by inhibiting the binding of BMP6 to its receptor [35]. Also, it was shown that membrane proteins, such as HFE and TfR2, trigger the ERK1/2 pathway which led to hepcidin expression [3637].
In conclusion, the current study demonstrates that iron transporter proteins in the duodenum, liver, and spleen are differentially regulated during developmental iron deficiency. Moreover, the post-weaning iron repletion efficiently restores iron transporters in the duodenum and the liver but not in the spleen, which suggests that the early-life iron deficiency may cause the long-term abnormalities in the iron recycling from the spleen.
Figures and Tables
 | Fig. 1Effects of developmental iron deficiency and the post-weaning iron repletion on the protein levels of ferritin and transferrin receptor (TfR) in the liver (A) and spleen (B).CON: control diet, ID: iron-deficient diet, IDR: iron-deficient diet followed by control diet. Upper panels: representative blots. Lower panels: Densitometric analyses. Data are means ± SEM, n = 6-8/group. Different superscript means significant difference, P < 0.05.
|
 | Fig. 2Effects of developmental iron deficiency and the post-weaning iron repletion on the mRNA levels of hepcidin (A) and BMP6 (B).CON: control diet, ID: iron-deficient diet, IDR: iron-deficient diet followed by control diet. β-actin was used as the housekeeping gene. Data are means ± SEM, n = 6-8/group. Different superscript means significant difference, P < 0.05.
|
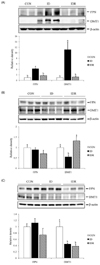 | Fig. 3Effects of developmental iron deficiency and the post-weaning iron repletion on the protein levels of FPN and DMT1 expression in the duodenum (A), liver (B), and spleen (C).CON: control diet, ID: iron-deficient diet, IDR: iron-deficient diet followed by control diet. Upper panels: representative blots. Lower panels: Densitometric analyses. Data are means ± SEM, n = 6-8/group. Different superscript means significant difference, P < 0.05.
|
References
1. Brigham D, Beard J, Tobin B. Iron and thermoregulation: a review. Crit Rev Food Sci Nutr. 1996; 36:747–763.

2. Lozoff B, Brittenham GM. Behavioral aspects of iron deficiency. Prog Hematol. 1986; 14:23–53.
3. Falkingham M, Abdelhamid A, Curtis P, Fairweather-Tait S, Dye L, Hooper L. The effects of oral iron supplementation on cognition in older children and adults: a systematic review and meta-analysis. Nutr J. 2010; 9:4.

4. Lozoff B. Early iron deficiency has brain and behavior effects consistent with dopaminergic dysfunction. J Nutr. 2011; 141:740S–746S.

5. Greminger AR, Mayer-Pröschel M. Identifying the threshold of iron deficiency in the central nervous system of the rat by the auditory brainstem response. ASN Neuro. 2015; 7.

6. Tran PV, Fretham SJ, Wobken J, Miller BS, Georgieff MK. Gestationalneonatal iron deficiency suppresses and iron treatment reactivates IGF signaling in developing rat hippocampus. Am J Physiol Endocrinol Metab. 2012; 302:E316–E324.

7. Chung J, Wessling-Resnick M. Molecular mechanisms and regulation of iron transport. Crit Rev Clin Lab Sci. 2003; 40:151–182.

8. Gulec S, Anderson GJ, Collins JF. Mechanistic and regulatory aspects of intestinal iron absorption. Am J Physiol Gastrointest Liver Physiol. 2014; 307:G397–G409.

9. Sharp PA. Intestinal iron absorption: regulation by dietary & systemic factors. Int J Vitam Nutr Res. 2010; 80:231–242.
10. Shah YM, Matsubara T, Ito S, Yim SH, Gonzalez FJ. Intestinal hypoxiainducible transcription factors are essential for iron absorption following iron deficiency. Cell Metab. 2009; 9:152–164.

11. Srai SK, Chung B, Marks J, Pourvali K, Solanky N, Rapisarda C, Chaston TB, Hanif R, Unwin RJ, Debnam ES, Sharp PA. Erythropoietin regulates intestinal iron absorption in a rat model of chronic renal failure. Kidney Int. 2010; 78:660–667.

12. Hoki T, Miyanishi K, Tanaka S, Takada K, Kawano Y, Sakurada A, Sato M, Kubo T, Sato T, Sato Y, Takimoto R, Kobune M, Kato J. Increased duodenal iron absorption through up-regulation of divalent metal transporter 1 from enhancement of iron regulatory protein 1 activity in patients with nonalcoholic steatohepatitis. Hepatology. 2015; Forthcoming.

13. Galy B, Ferring-Appel D, Becker C, Gretz N, Gröne HJ, Schümann K, Hentze MW. Iron regulatory proteins control a mucosal block to intestinal iron absorption. Cell Rep. 2013; 3:844–857.

14. Bhaskaram P, Balakrishna N, Radhakrishna KV, Krishnaswamy K. Validation of hemoglobin estimation using Hemocue. Indian J Pediatr. 2003; 70:25–28.

15. Ahmed BS, Ahmed SN, Hassan HG. Ferritic as a potent marker of brest cancer. Int J Tech Res Appl. 2015; 3:184–187.
16. Torrance JD, Bothwell TH. A simple technique for measuring storage iron concentrations in formalinised liver samples. S Afr J Med Sci. 1968; 33:9–11.
17. Sim MO, Lee HI, Ham JR, Seo KI, Kim MJ, Lee MK. Anti-inflammatory and antioxidant effects of umbelliferone in chronic alcohol-fed rats. Nutr Res Pract. 2015; 9:364–369.

18. Min B, Lee H, Song JH, Han MJ, Chung J. Arctiin inhibits adipogenesis in 3T3-L1 cells and decreases adiposity and body weight in mice fed a high-fat diet. Nutr Res Pract. 2014; 8:655–661.

19. Lee SM, Lee SB, Prywes R, Vulpe CD. Iron deficiency upregulates Egr1 expression. Genes Nutr. 2015; 10:468.

20. Cairo G, Tacchini L, Pogliaghi G, Anzon E, Tomasi A, Bernelli-Zazzera A. Induction of ferritin synthesis by oxidative stress. Transcriptional and post-transcriptional regulation by expansion of the "free" iron pool. J Biol Chem. 1995; 270:700–703.
21. Dongiovanni P, Lanti C, Gatti S, Rametta R, Recalcati S, Maggioni M, Fracanzani AL, Riso P, Cairo G, Fargion S, Valenti L. High fat diet subverts hepatocellular iron uptake determining dysmetabolic iron overload. PLoS One. 2015; 10:e0116855.

22. Zhang AS, Enns CA. Molecular mechanisms of normal iron homeostasis. Hematology Am Soc Hematol Educ Program. 2009; 207–214.

23. Montalbetti N, Simonin A, Simonin C, Awale M, Reymond JL, Hediger MA. Discovery and characterization of a novel non-competitive inhibitor of the divalent metal transporter DMT1/SLC11A2. Biochem Pharmacol. 2015; 96:216–224.

24. Ikuta K, Yersin A, Ikai A, Aisen P, Kohgo Y. Characterization of the interaction between diferric transferrin and transferrin receptor 2 by functional assays and atomic force microscopy. J Mol Biol. 2010; 397:375–384.

27. Nemeth E. Targeting the hepcidin-ferroportin axis in the diagnosis and treatment of anemias. Adv Hematol. 2010; 2010:750643.

28. De Domenico I, Ward DM, Langelier C, Vaughn MB, Nemeth E, Sundquist WI, Ganz T, Musci G, Kaplan J. The molecular mechanism of hepcidin-mediated ferroportin down-regulation. Mol Biol Cell. 2007; 18:2569–2578.

29. De Domenico I, Lo E, Ward DM, Kaplan J. Hepcidin-induced internalization of ferroportin requires binding and cooperative interaction with Jak2. Proc Natl Acad Sci U S A. 2009; 106:3800–3805.

30. Tsochatzis E, Papatheodoridis GV, Koliaraki V, Hadziyannis E, Kafiri G, Manesis EK, Mamalaki A, Archimandritis AJ. Serum hepcidin levels are related to the severity of liver histological lesions in chronic hepatitis C. J Viral Hepat. 2010; 17:800–806.

31. Xia Y, Babitt JL, Sidis Y, Chung RT, Lin HY. Hemojuvelin regulates hepcidin expression via a selective subset of BMP ligands and receptors independently of neogenin. Blood. 2008; 111:5195–5204.

32. Kautz L, Meynard D, Monnier A, Darnaud V, Bouvet R, Wang RH, Deng C, Vaulont S, Mosser J, Coppin H, Roth MP. Iron regulates phosphorylation of Smad1/5/8 and gene expression of Bmp6, Smad7, Id1, and Atoh8 in the mouse liver. Blood. 2008; 112:1503–1509.

33. Rausa M, Pagani A, Nai A, Campanella A, Gilberti ME, Apostoli P, Camaschella C, Silvestri L. Bmp6 expression in murine liver non parenchymal cells: a mechanism to control their high iron exporter activity and protect hepatocytes from iron overload? PLoS One. 2015; 10:e0122696.

34. Andriopoulos B Jr, Corradini E, Xia Y, Faasse SA, Chen S, Grgurevic L, Knutson MD, Pietrangelo A, Vukicevic S, Lin HY, Babitt JL. BMP6 is a key endogenous regulator of hepcidin expression and iron metabolism. Nat Genet. 2009; 41:482–487.

35. Fung E, Nemeth E. Manipulation of the hepcidin pathway for therapeutic purposes. Haematologica. 2013; 98:1667–1676.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download