INTRODUCTION
MATERIALS AND METHODS
Smilax china L. leaf extracts
Determination of total phenolic and total flavonoid contents
Cytotoxicity assays and induction of 3T3-L1 cell differentiation
Measuring glycerol release
Cyclic AMP assay
Western blot analysis
Statistical analysis
RESULTS
Total phenolic content and cell viability of the water-soluble fraction of Smilax china L. leaf ethanol extract (wsSCLE)
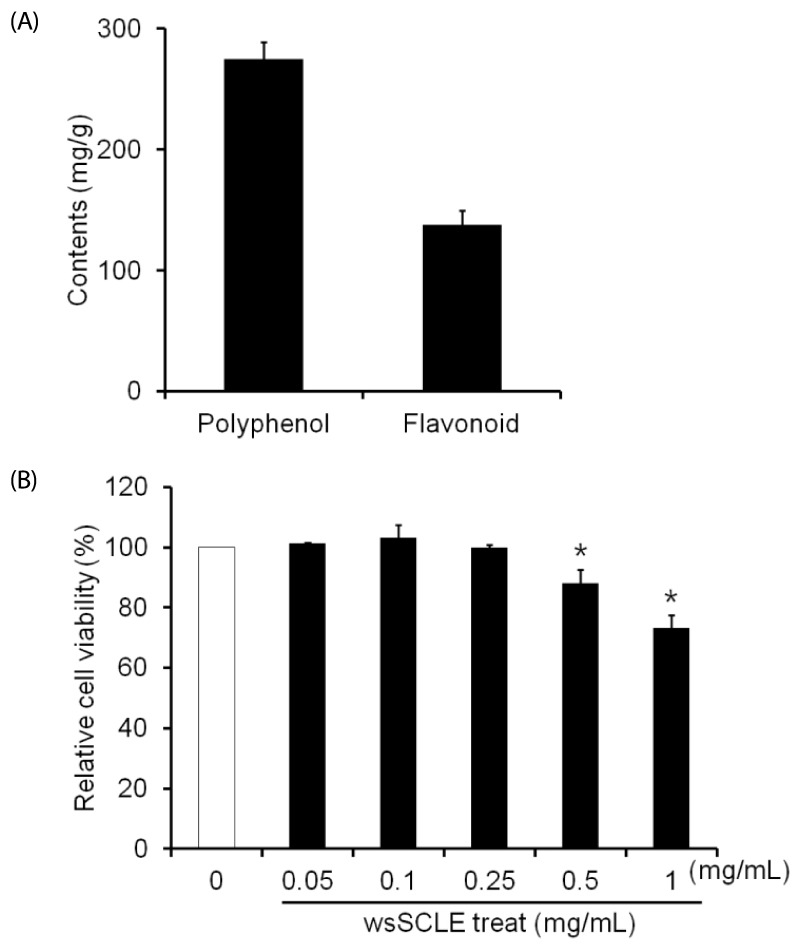 | Fig. 1Total phenolic content of a water-soluble fraction of Smilax china L. leaf ethanol extract (wsSCLE) and its effects on cell viability.(A) The total phenolic and total flavonoid contents were expressed as mg of gallic acid and quercetin equivalents per g of dry extract. (B) Effect of wsSCLE on the viability of 3T3-L1 cells. Cells were treated with wsSCLE at 0.05, 0.1, 0.25, 0.5, or 1 mg/mL for 24 h. Cell viability was determined using a MTT assay kit. Data are expressed as mean ± SD of triplicate experiments, *
P < 0.05 compared to control cells (white bar).
|
Inhibitory effects of wsSCLE on lipid accumulation
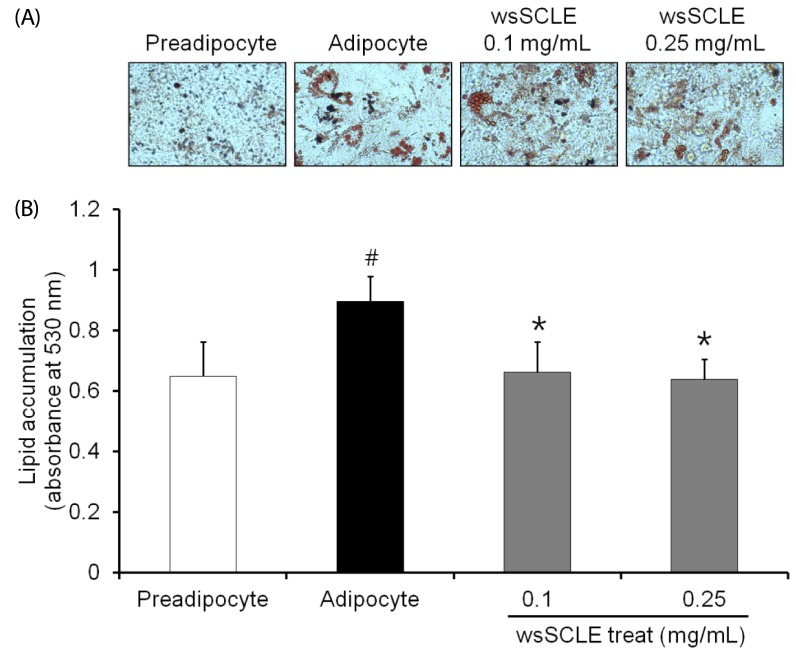 | Fig. 2A water-soluble fraction of Smilax china L. leaf ethanol extract (wsSCLE) treatment inhibits lipid accumulation in 3T3-L1 adipocytes.Differentiation was induced in 3T3-L1 cells 2 days after they attained confluence. Cells were then treated with wsSCLE (0.1 and 0.25 mg/mL) for 9 days. At the end of the treatment period, lipid content was analyzed using Oil Red O staining. (A) Representative images of Oil Red O staining. (B) Quantification of lipid accumulation based on the optical density values at 520 nm of destained Oil Red O extracted from the 3T3-L1 cells. Data are expressed as mean ± SD of triplicate experiments. #
P < 0.05 compared to control cells (white bar), *
P < 0.05 compared to adipocytes.
|
Effects of wsSCLE on lipolysis in 3T3-L1 adipocytes
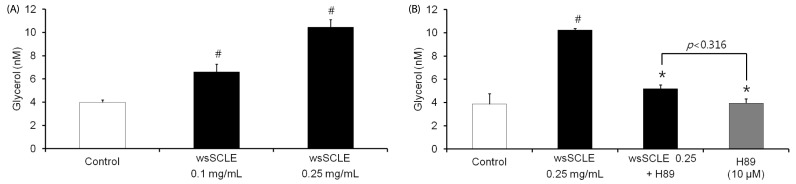 | Fig. 3Effects of a water-soluble fraction of Smilax china L. leaf ethanol extract (wsSCLE) treatment on lipolysis in mature 3T3-L1 adipocytes.(A) wsSCLE (0.1 and 0.25 mg/mL) increased glycerol release from differentiated mature 3T3-L1 cells. (B) Lipolytic effect of wsSCLE was inhibited by treatment with the protein kinase A (PKA) inhibitor (H89, 10 µM). After differentiation, the mature 3T3-L1 cells were incubated with wsSCLE. Glycerol release was measured using a commercial glycerol assay kit. Data are expressed as mean ± SD of triplicate experiments, #
P < 0.05 compared to control cells (white bar), *
P < 0.05 compared to wsSCLE 0.25 mg/mL.
|
The effect of wsSCLE on the expression of adenylate cyclase (AC) and PKA
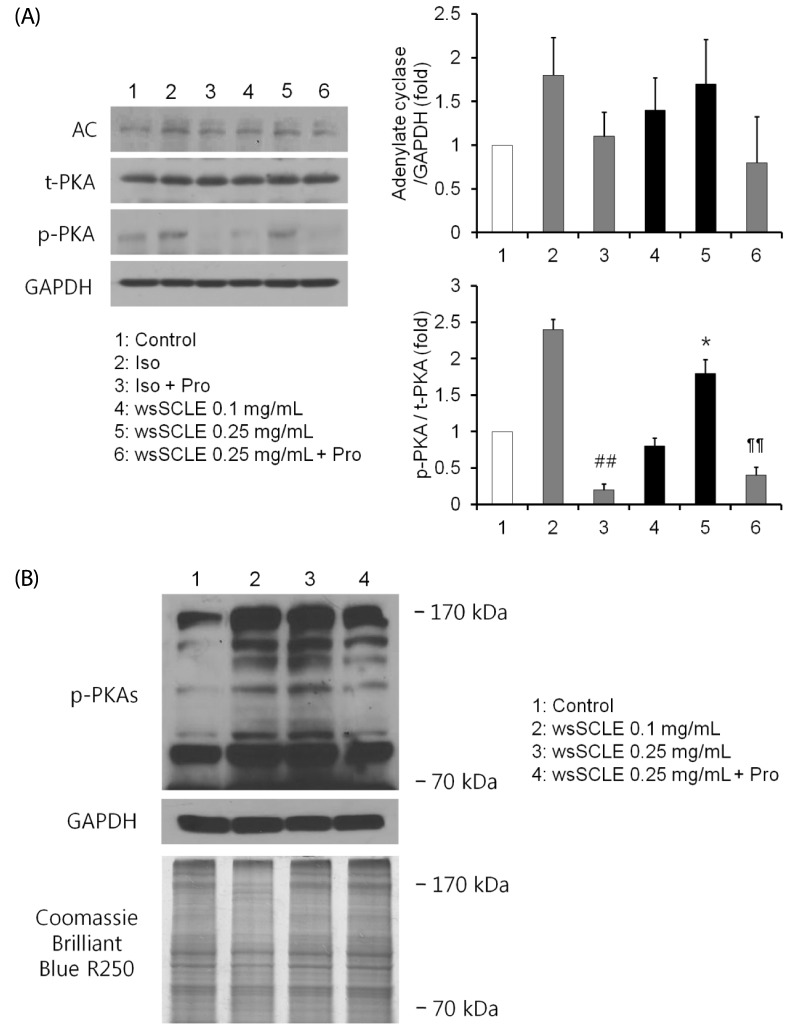 | Fig. 4A water soluble fraction of Smilax china L. leaf ethanol extract (wsSCLE) stimulates protein kinase A (PKA) signaling.(A) The expression of adenylate cyclase (AC) and PKA by wsSCLE treatment. Mature 3T3-L1 adipocytes were incubated with wsSCLE (0.1 and 0.25 mg/mL) for 24 h. The β-adrenergic receptors were induced (by Iso, an agonist) and inhibited (by Pro, an antagonist). (B) The induction of the phosphorylation (p) of PKA substrates (PKAs) by wsSCLE treatment. H89 treatment (10 µM) decreased wsSCLE-induced pPKAs. Data are expressed as mean ± SD of triplicate experiments, ##
P < 0.001 compared to Iso, *
P < 0.05 compared to control (white bar), ¶¶
P < 0.001 compared to wsSCLE 0.25 mg/mL. Iso, isoproterenol (10 µM); Pro, propranolol (100 µM).
|
Effects of wsSCLE on cAMP synthesis
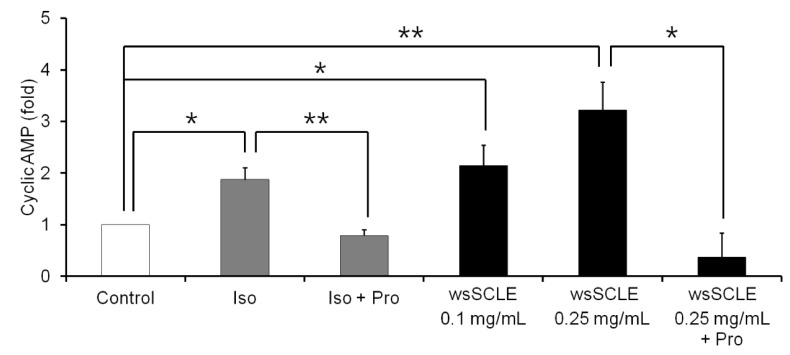 | Fig. 5A water-soluble fraction of Smilax china L. leaf ethanol extract (wsSCLE) induces lipolysis via activation of the cyclic adenosine monophosphate (cAMP) signaling.Mature 3T3-L1 adipocytes were incubated with wsSCLE (0.1 and 0.25 mg/mL) for 24 h. A β-adrenergic receptor agonist and antagonist (Iso and Pro, respectively) were used as cAMP inducer and inhibitor, respectively. Intracellular cAMP levels were measured using an enzyme-linked immunosorbent assay (ELISA). Data are expressed as mean ± SD of triplicate experiments. *
P < 0.05, **
P < 0.001. Iso, isoproterenol (10 µM); Pro, propranolol, (100 µM).
|
Effects of wsSCLE on the expression of HSL and phosphorylation
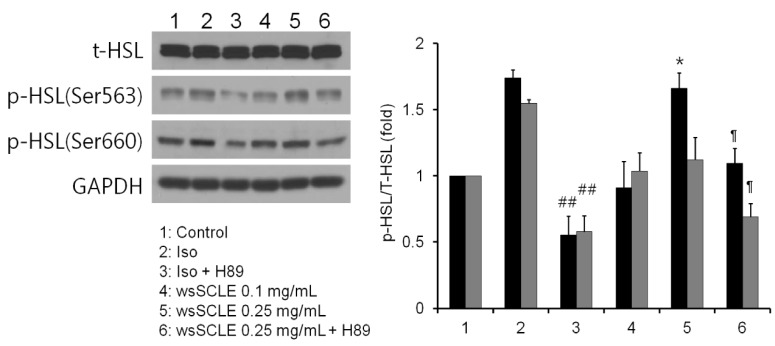 | Fig. 6A water-soluble fraction of Smilax china L. leaf ethanol extract (wsSCLE) induces phosphorylation of hormone-sensitive lipase (HSL).Mature 3T3-L1 adipocytes were incubated with wsSCLE (0.1 and 0.25 mg/mL) for 24 h. The β-adrenergic receptor agonist and antagonist (Iso and Pro, respectively) were used to induce phosphorylation and dephosphorylation, respectively, of HSL. Data are expressed as mean ± SD of triplicate experiments, ##
P < 0.001 compared to Iso,*
P < 0.05 compared to control, ¶
P < 0.05 compared to wsSCLE 0.25 mg/mL. Iso, isoproterenol (10 µM); Pro, propranolol (100 µM).
|




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download