Abstract
BACKGROUND
Currently, natural products have been shown to exhibit interesting biological and pharmacological activities and are used as chemotherapeutic agents. The purpose of this study, conducted on Wistar rats, was to evaluate the beneficial effects of Artemisia arborescens oil on oestroprogestative treatment induced damage on liver.
MATERIALS/METHODS
A total of 36 Wistar rats were divided into 4 groups; a control group (n = 9), a group of rats who received oestroprogestative treatment by intraperitoneal injection (n = 9), a group pre-treated with Artemisia arborescens then injected with oestroprogestative treatment (n = 9), and a group pre-treated with Artemisia arborescens (n = 9). To minimize the handling stress, animals from each group were sacrificed rapidly by decapitation. Blood serum was obtained by centrifugation and the livers were removed, cleaned of fat, and stored at -80℃ until use.
RESULTS
In the current study, oestroprogestative poisoning resulted in oxidative stress, which was demonstrated by 1) a significant increase of lipid peroxidation level in hepatic tissue 2) increased levels of serum transaminases (aspartate amino transferase and serum alanine amino transferase), alkaline phosphatase, glycemia and triglycerides and a decrease in the level of cholesterol 3) alteration of hepatic architecture. Pre-administration of Artemisia arborescens oil was found to alleviate oestroprogestative treatment induced damage by lowering lipid peroxidation level and by increasing activity of catalase, superoxide-dismutase, and glutathione-peroxidase in liver and by reducing disruption of biochemical parameters.
Drug-induced liver injury can be defined as liver injury caused by drugs or herbal medicines, leading to liver test abnormalities or liver dysfunction with reasonable exclusion of other etiologies. More than 900 drugs have been implicated in causing liver injury [1]. In fact, chemicals often cause subclinical injury to the liver, which is manifested only as abnormal liver enzyme tests. Drug-induced liver injury is responsible for 5% of all hospital admissions and 50% of all cases of acute liver failure. In recent years, chronic drug administration has become a major cause of liver damage. It is also the main reason for the withdrawal of drugs from the market and cessation of new drug development [2].
Estradiol and progesterone, the most important gonadal steroids hormones in females, exert various physiological actions throughout the body. However, in addition to the classic role of hormones in reproduction, their toxicity properties have become a major focus over the last decade [3]. Indeed, many research studies have suggested that the frequent use of hormones can disrupt liver metabolism by elevation of triglycerides. The latter may aggravate the pre-existing hypertriglyceridemia and, sometimes, produce marked hyperlipidemia [4].
Numerous research studies have also reported that although sex steroids are critical for development and function of the normal human, long-term exposure to them increases the risk of oxidative stress. This is a primary cause of development of aging and diseases such as inflammation, infection, cancer, and cardiovascular disorders. It also weakens the antioxidative defenses, which can lead to damage of macromolecules such as DNA and proteins [5].
Current evidence suggests that oxidative stress with the
increased generation of reactive oxygen species, depletion of reduced glutathione (GSH) and lipid peroxidation play an essential role in development of oestroprogestative administration (combination of estrogen and progesterone) induced liver damage [6]. Several antioxidants were proven to have protective effects against liver damage and the antioxidant capacity is widely used as a parameter for characterization of nutritional plants and their bioactive components [7]. Thus, recent studies have investigated the potential of plant products as antioxidants against various diseases induced by free radicals [8]. Artemisia arborescens was proven successful in protecting organs against oxidative stress in various experiments.
Artemisia arborescens is an aromatic plant of the family Asteraceae, distributed in Europe, South Africa, and Asia. It is not only widely used as a traditional medicine but is also known to possess ethnomedical and biological properties related to antiviral [9], antifungal, anticoagulant, hypoglycemic [10], and antispasmodic activities [11]. Artemisia arborescens essential oil has been used in treatment of inflammation, intestinal trouble, and diarrhea [12]. Indeed, high efficiency of Artemisia essential oil against free radicals has been confirmed [13]. In addition, a number of studies have demonstrated that the antioxidant effect of the essential oil is mainly attributed to their components [14]. The current study was designed to evaluate the hepatoprotective effect of Artemisia essential oil against oestroprogestative treatment toxicity on female rats. First, some biochemical parameters, including alanine aminotransferase (ALAT), lactate dehydrogenase (LDH), aspartate aminotransferase (ASAT), and alkalinephosphatase (ALP) activities were measured in blood serum. In addition, lipid peroxidation level and antioxidant enzyme (SOD, GPX, and CAT) activities were determined in liver. An analysis of GC-Ms of Artemesia was performed for identification of its chemical composition.
The aerial parts of Artemisia arborescens were collected from a rural area around Gafsa, Tunisia, with the help of a botanist at the department of Biology University of Gafsa. The material was cut into small pieces and subjected to hydrodistillation.
Essential oils were extracted by hydrodistillation of dried plant material (100 g of Artemisia in 500 ml of distilled water) using a Clevenger-type apparatus for 4.5 h, as described in the European Pharmacopeia. The essential oil was collected, dried under anhydrous sodium sulphate, and stored at 4℃ until future use.
At total of 36 Wistar female rats (3-months-old), approximately 160 g body weight, obtained from the Animal Ecophysiology Laboratory (Sfax, Tunisia), were maintained for a two-week adaptation period under the same conditions of temperature (22 ± 2℃), relative humidity (50 ± 4%), and a constant photoperiod (12 h light/dark cycle). Animals were fed pellets (SICO, Sfax, Tunisia) [composition: corn, Luzerne, Soja, CMV (Compound Mineral Vitaminized), Dicalcium phosphate, lime carbonate, Salt-pure methionine, Trace Elements] Table 1. The animals were treated according to the Tunisian code of practice for the Care and Use of Animals for Scientific Purposes and the European convention for the protection of vertebrate animals used for experimental and other scientific purposes (Council of Europe No123, Strasbourg, 1985).
After the adaptation period, the animals were placed in 4 groups, with 9 rats in each group; treatment was then administered as follows:
Group 1 (C) served as the control group.
Group 2 (E) was given daily a combination of estradiol and progesterone (i.p intraperitoneal injection) during a period of 42 days [50 µg/kg of estradiol (17-Ethynylestradiol) and 125 µg/kg of progesterone/body weight.
Group 3 (AE) received oestroprogestative treatment and the essential oil of Artemisia (20 mg/kg body weight/day) before 2 hours of oestroprogestative administration.
Group 4 (A) received only essential oil of Artemisia (20 mg/kg body weight/day) (i.p) daily during a period of 6 weeks.
After 6 weeks from the start of the treatment, animals from each group were sacrificed rapidly by decapitation to avoid the effect of stress, which induces the unloading of hyperglycemic hormones: catecholamines and glucocorticoids.
Blood serum was obtained by centrifugation (1500 × g, 15 min, 4℃), and the livers were removed, cleaned of fat, and stored at -80℃ until use.
1 g of the organ, cut out in small pieces, was homogenized in 2 ml of tris buffer solution (TBS) (pH 7.4) using a crusher (homogenizing Ultra-Turax); the homogenate was then centrifuged with 9000 tr/min for 15 min in cold (4℃). The supernatants were collected and stored at -80℃ until use.
The level of lipid peroxidation was measured as thiobarbituric acid reactive substances (TBARS), according to Yagi's method [15]. For the assay, 125 µl of supernatant (S1) were mixed with 175 µl of 20% trichloroacetic acid containing 1% butyl-hydroxytoluene and centrifuged (1000 × g, 10 min, 4℃). Then, 200 µl of supernatant (S2) was mixed with 40 µl of HCl (0.6 M) and 160 µl of thiobarbituric acid (0.72 mM) and the mixture was heated at 80℃ for 10 min. Absorbance was measured at 530 nm. The amount of TBARS was calculated using an extinction coefficient of 156 mM-1 cm-1 and expressed as nmoles/mg protein.
The total superoxide-dismutase (SOD) activity was determined by measuring its ability to inhibit the photoreduction of nitrobluetetrazolium (NBT) [16]. One unit of SOD represents the amount inhibiting the photoreduction of NBT by 50%. The activity is expressed as units/mg protein, at 25℃.
Glutathione-peroxidase (GPX) activity was assayed according to the method of Flohe and Gunzler [17]. The activity at 25℃ was expressed as µmoles of GSH oxidized/min/g protein.
Catalase (CAT) activity was measured according to the method of Aebi [18]. The reaction mixture (1 ml) contained 100 mM phosphate buffer (pH = 7), 100 mM H2O2, and 20 µl (approximately 1-1.5 mg of protein) of liver. H2O2 decomposition was determined at 25℃ by measuring the decrease in absorbance at 240 nm for 1 min. The enzyme activity was calculated using an extinction coefficient of 0.043 mM-1 cm-1 and expressed in international units (I.U.) i.e., in µmoles H2O2 destroyed/min/mg protein.
Protein content in tissue extracts was determined according to Lowry's method [19] using bovine serum albumin as standard.
The levels of glucose, cholesterol, triglycerides and the activity of alkaline phosphatase, lactate dehydrogenase (LDH), aspartate amino transferase (AST), and alanine amino transferase (ALT) in serum, were determined using kit methods (Spinreact).
Portions were fixed in 10% formalin, processed through tap water then a graded alcohol series and finally xylol, and embedded in paraffin. Six-micrometer-thick tissue sections were prepared and stained with hematoxylin-eosin (HE) and examined under a light microscope. [20].
The essential oils were analyzed using an Agilent-Technologies 6890 N Network GC system equipped with a flame ionization detector and HP-5MS capillary column (30 m × 0.25 mm, film thickness 0.25 µm; Agilent-Technologies, Little Falls, CA, USA). The injector and detector temperatures were set at 220 and 290℃, respectively. The column temperature was programmed from 80 to 220℃ at a rate of 4℃/min, with the lower and upper temperatures being held for 3 and 10 min, respectively. The flow rate of the carrier gas (Helium) was 1.0 ml/min. A sample of 1.0 µl was injected, using split mode (split ratio, 1:100). All quantifications were performed using a built-in data-handling program provided by the manufacturer of the gas chromatograph. The identity of the components was determined by comparison of their retention indices, relative to n-alkanes indices and GC-MS spectra from a home-made library, constructed on the basis of the analysis of reference oils, laboratory-synthesized components and commercially available standards.
Oestroprogestative treatment induced severe liver damage evidenced in serum by a significant increase of glycaemia, AST, ALT, triglycerides, LDH, and decrease of cholesterol (Table 2); Pre-administration of Artemisia arborescens oil was found to alleviate oestroprogestative treatment resulted in restoration of all these biomarkers to almost normal values.
TBARS levels in hepatic tissues were increased in the group that received oestroprogestative treatment as compared to controls by +84% (Fig. 1). Administration of Artemisia arborescens essential oil resulted in significant reduction of these TBARS levels to normal.
Activities of enzymes that protect against oxidative stresses, i.e., SOD, CAT, and GPX, were respectively reduced by -55%, -28%, and -47% in liver of group (E), as compared to controls (Fig. 1). These changes, indicating a failing defense against an oxidative stress, were largely corrected in animals treated by Artemisia arborescens.
Light microscopic observations revealed the regular morphology of the liver tissue with well-designed hepatic cells and sinusoids in the control group (Fig. 2A). When compared to control liver sections, the histology of the liver of rats treated with estrogen and progesterone showed cortico-inflammatory lesions, gross necrosis along with cytoplasmic vacuolation around the nuclei and hyperchromatia (Fig. 2B). These alterations were attenuated in the group pre-treated with oil of Artemisia at 20 mg/kg dose (oestroprogestative administration plus Artemisia) (Fig. 2C). In fact, in comparison with the treated specimens, hepatic sections demonstrated protection in the histopathological pattern of the liver with better cord arrangement of well-formed polygonal hepatocytes and relatively reduced cytoplasmic vacuolation. Rats treated with essential oil of Artemisia had normal liver structure (Fig. 2D).
The results obtained in the GC/MS analysis of the oil are shown in Table 3. The essential oil was found to contain 22 constituents representing 99.22% of the total essential oil while minor constituents (0.78%) of the oil remained unidentified. The color of the essential oil from Artemisia arborescens L. aerial part is blue and the yield of hydrodistillation is 0.87% (w/w) in relation to the dry weight of the plant. The main constituents were chamazulene (33.4%), camuphor (26.87%), Boronylacetate (6.34%), and 4-Carvomenthenol (4.87%).
Although the protective effect of Artemisia arborescens on the antioxidant defense has been studied extensively, there are a few data on the protective use of its essential oil on liver damage. As a major site of xenobiotic metabolism, liver can be altered by combined hormonal contraception. In the current study, damage to liver was evidenced by hyperglycemia, increased levels of triglycerides AST, ALAT, and LDH activities, and a decreased level of cholesterol in blood.
AST, and more particularly ALT, which are released into the blood when the liver is injured, are commonly used in diagnosis of hepatocellular injury [21]. The increased levels of transaminases and the histopathological observations were noted after administration of contraception. In addition, the same treatment increased the level of glycaemia by approximately 17% in blood, indicating liver damage. Similar results were reported by Yamamoto et al. [22], who confirmed that the mechanism of these changes is related to the ability of estrogen to produce oxidative stress [23]. Under the experimental conditions, the disrupted liver function occurring in rats treated with oestroprogestative administration can be attributed to general oxidative stress represented by an increase of lipid peroxidation, the marker of oxidative damage. A decrease of (SOD, GPx, and CAT) activities was determined as compared to controls. This supports the idea that oxidative stress and overproduction of reactive oxygen species (ROS) are a part of the mechanism of oestroprogestative treatment toxicity; it can also be explained by the fact that metabolic redox cycling between catechol estrogens and their corresponding quinones generates oxidative stress and potentially harmful free radicals [24].
The results obtained in this study support and extend those of previous reports suggesting that steroid hormone intoxication generally causes impairment of the hepatic antioxidant defense system and induces lipid peroxidation in experimental animals [2526]. They also provide strong evidence that Artemisia can significantly inhibit liver toxicity induced by hormonal contraception in rat. Actually, all measured parameters (glycaemia, triglycerides, cholesterol, ALAT, ASAT, ALP and LDH, SOD, GPX and CAT) were restored to almost normal values, which can be explained by two reasons. First, the modulation of the cellular GSH pool was found to improve the antioxidant activity for scavenging free radicals. Second, the components of Artemisia appear to be able to counteract the oestroprogestative administration induced production of aggressive oxidants. They can also impair the mechanisms by which these oxidants damage the key molecules within the tissues [27]. The antioxidant properties of Artemisia were attributed to flavonoids, β-Caryophyllene, α-thujone, Boronyl acetate, and phospholipids, which are all known to be efficient radical scavengers [28]. The observed reduction in the level of lipid peroxidation in the group of animals pre-treated with Artemisia is in due in part to its ability to scavenge hydroxyl and peroxyl radicals [2930]. These findings are in agreement with those of studies reporting that Artemisia oil might play a key role in protection against oestroprogestative treatment intoxication by reducing lipid peroxidation in the rat liver through its antioxidant effects [31].
Lai et al. [32] demonstrated that the antioxidant activity of the essential oil could be attributed in part to the presence of compounds such as chamazulene and camuphor and its ability to decompose free radicals by quenching reactive oxygen species and trapping radicals before reaching their cellular targets. Our results show that the essential oil of Artemisia arborescens could exert in vivo an antioxidant activity by hindering the lipid peroxidation process, restoring GSH depletion and reversing redox imbalance induced toxicity of oestroprogestative administration. Further preclinical studies and clinical trials in humans are needed in order to find a possible place for essential oil of Artemisia arborescens in therapies for drug toxicity.
Figures and Tables
Fig. 1
Levels of lipid peroxidation (expressed as TBARS, nmol/mg protein) and activities (I.U/mg protein) of superoxide dismutase (SOD), glutathione peroxidase (GPX), and catalase (CAT) in liver after 6 weeks of treatment.
C: control group; E: treated group with estradiol and progesterone; AE: treatad group previously supplemented with Artemisia; A: supplemented with essential oil. the percent number in graph is the percent of difference between the mean values of two groups. Values are expressed as the mean of 9 measurements ± SD. aP ≤ 0.05 vs. treated group (E). bP ≤ 0.05 vs. control group (C). c P ≤ 0.05 vs. treated and supplemented with Artemisia arborescens group (AE). d P ≤ 0.05 vs. supplemented with Artemisia arborescens group (A)

Fig. 2
Microscopic observations of rat liver sections (H&E, A, B, C and D: 400×).
(A) control group showing normal hepatic architecture. (B) oestroprogestative-Treated group showing cortico-inflammatory lesions and hyperchromatia. (C) rats received combination of oestroprogestative treatment and Artemisia oil showing marked improvement in the histological sections with well-formed polygonal hepatocytes. (D) artemisia treated group have normal strutcture similar to control.
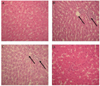
Table 2
Effects of oestroprogestative treatment and Artemisia arborescens essential oil on serum markers of liver

C: normal, E: oestroprogestative treatment, AE: oestroprogestative treatment + artemisia, A: artemisia, ALP: alkalinephosphatase, LDH: lactate dehydrogenase, ASAT: aspartate aminotransferase, ALAT: alanine aminotransferase.
Values are expressed as the mean of 9 measurements ± SD.
a P ≤ 0.05 vs. treated group (E).
b P ≤ 0.05 vs. control group (C).
c P ≤ 0.05 vs. treated and supplemented with Artemisia arborescens group (AE).
d P ≤ 0.05 vs. supplemented with Artemisia arborescens group (A)
References
1. Mladenović D, Radosavljević T, Ninković M, Vucević D, Jesić-Vukićević R, Todorović V. Liver antioxidant capacity in the early phase of acute paracetamol-induced liver injury in mice. Food Chem Toxicol. 2009; 47:866–870.

2. Potts RO, Lobo RA. Transdermal drug delivery: clinical considerations for the obstetrician-gynecologist. Obstet Gynecol. 2005; 105:953–961.

3. Elouni B, Ben Salem C, Zamy M, Ganne N, Beaugrand M, Bouraoui K, Biour M. Cytolytic hepatitis possibly related to levonorgestrel/ethinylestradiol oral contraceptive use: 2 case reports. Ann Pharmacother. 2010; 44:2035–2037.

4. Huang L, Smit JW, Meijer DK, Vore M. Mrp2 is essential for estradiol-17beta(beta-D-glucuronide)-induced cholestasis in rats. Hepatology. 2000; 32:66–72.

5. Sitruk-Ware RL, Menard J, Rad M, Burggraaf J, de Kam ML, Tokay BA, Sivin I, Kluft C. Comparison of the impact of vaginal and oral administration of combined hormonal contraceptives on hepatic proteins sensitive to estrogen. Contraception. 2007; 75:430–437.

6. Goldfarb S. Sex hormones and hepatic neoplasia. Cancer Res. 1976; 36:2584–2588.
7. Rezaeinodehi A, Khangholi S. Chemical composition of the essential oil of Artemisia absinthium growing wild in Iran. Pak J Biol Sci. 2008; 11:946–949.

8. Ghasemi K, Ghasemi Y, Ebrahimzadeh MA. Antioxidant activity, phenol and flavonoid contents of 13 citrus species peels and tissues. Pak J Pharm Sci. 2009; 22:277–281.
9. Lee HW, Ko YH, Lim SB. Effects of selected plant extracts on anti-oxidative enzyme activities in rats. Food Chem. 2012; 132:1276–1280.

10. Kordali S, Cakir A, Mavi A, Kilic H, Yildirim A. Screening of chemical composition and antifungal and antioxidant activities of the essential oils from three Turkish artemisia species. J Agric Food Chem. 2005; 53:1408–1416.

11. Presti ML, Crupi ML, Zellner BD, Dugo G, Mondello L, Dugo P, Ragusa S. Characterization of Artemisia arborescens L. (Asteraceae) leaf-derived essential oil from Southern Italy. J Essent Oil Res. 2007; 19:218–224.

12. Sharopov FS, Sulaimonova VA, Setzer WN. Composition of the essential oil of Artemisia absinthium from Tajikistan. Rec Nat Prod. 2012; 6:127–134.
13. Lopes-Lutz D, Alviano DS, Alviano CS, Kolodziejczyk PP. Screening of chemical composition, antimicrobial and antioxidant activities of Artemisia essential oils. Phytochemistry. 2008; 69:1732–1738.

14. Ghasemi Pirbalouti A, Firoznezhad M, Craker L, Akbarzadeh M. Essential oil compositions, antibacterial and antioxidant activities of various populations of Artemisia chamaemelifolia at two phenological stages. Rev Bras Farmacogn. 2013; 23:861–869.

15. Yagi K. A simple fluorometric assay for lipoperoxide in blood plasma. Biochem Med. 1976; 15:212–216.

16. Sun Y, Oberley LW, Li Y. A simple method for clinical assay of superoxide dismutase. Clin Chem. 1988; 34:497–500.

17. Flohé L, Günzler WA. Assays of glutathione peroxidase. Methods Enzymol. 1984; 105:114–121.
18. Aebi H. Catalase in vitro. Methods Enzymol. 1984; 105:121–126.
19. Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951; 193:265–275.

20. Gabe M. Techniques Histologiques. Paris: Masson et Cie;1968.
21. Cay M, Ulas M. Effects of estrogen replacement therapy with vitamin E on oxidative stress in hepatic and pancreatic tissues of ovariectomized diabetic rats. J Anim Vet Adv. 2010; 9:2955–2962.

22. Yamamoto Y, Moore R, Hess HA, Guo GL, Gonzalez FJ, Korach KS, Maronpot RR, Negishi M. Estrogen receptor alpha mediates 17alpha-ethynylestradiol causing hepatotoxicity. J Biol Chem. 2006; 281:16625–16631.
23. Sabers A. Pharmacokinetic interactions between contraceptives and antiepileptic drugs. Seizure. 2008; 17:141–144.

24. Cavalieri EL, Rogan EG, Chakravarti D. Initiation of cancer and other diseases by catechol ortho-quinones: a unifying mechanism. Cell Mol Life Sci. 2002; 59:665–681.

25. O'Brien MD, Guillebaud J. Contraception for women taking antiepileptic drugs. J Fam Plann Reprod Health Care. 2010; 36:239–242.
26. Akash C, Kankanala VV, Ajit K, Nayana J, Naval C. Cytolytic hepatitis: a rare complication of oral contraceptives. J Clin Diagn Res. 2011; 5:1450–1451.
27. Judzentiene A, Tomi F, Casanova J. Analysis of essential oils of Artemisia absinthium L. from Lithuania by CC, GC(RI), GC-MS and 13C NMR. Nat Prod Commun. 2009; 4:1113–1118.

28. Lamharrar A, Idlimam A, Ethmane Kane CS, Jamali A, Abdenouri N, Kouhila M. Sorption isotherms and drying characteristics of Artemisia arborescens leaves. J Agron. 2007; 6:488–498.

29. Williams D. Contraception and prenatal vitamin supplementation for women on antiepileptic medications. Ment Health Clin. 2013; 3:88–95.

30. Abderrahim A, Belhamel K, Chalchat JC, Figuérédo G. Chemical composition of the essential oil from Artemisia arborescens L. growing wild in algeria. Rec Nat Prod. 2010; 4:87–90.
31. Mahmoudi M, Ebrahimzadeh MA, Ansaroudi F, Nabavi SF, Nabavi SM. Antidepressant and antioxidant activities of Artemisia absinthium L. at flowering stage. Afr J Biotechnol. 2009; 8:7170–7175.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download