Abstract
BACKGROUND/OBJECFTIVES
The effect of St. John's Wort extract (SJW) on MG-63 cell proliferation and trabecular bone loss induced by ovariectomy was examined.
MATERIALS/METHODS
Proliferation, expression of estrogen receptor (ER) α and ER β, and gene expressions of osteoprotegerin (OPG), osteocalcin (OC) and alkaline phosphatase (ALP) were examined in MG-63 cells treated with or without SJW. Ovariectomized rats were treated with SJW at the dose of 100 or 200 mg/kg/day, β-estradiol-3-benzoate (E2), or vehicle only (OVX-C), and sham operated rats were treated with vehicle only (Sham-C). Serum ALP and C-telopeptide (CTX), and femoral trabecular bone loss were examined.
RESULTS
SJW increased MG-63 cell proliferation and expression of ER α and ER β, and positive effect was shown on gene expressions of ALP, OC and OPG. SJW also showed estrogen like effect on bone associated with slowing down in trabecular bone loss. Histopathology by H&E showed rats treated with SJW displayed denser structure in metaphyseal region of distal femur compared with rats in OVX-C. SJW was shown to reduce serum CTX in OVX rats.
Osteoporosis is characterized by decreased bone mass and loss of microarchitectural integrity, due to an imbalance between bone resorption and bone formation [1]. The decrease in bone loss increases the risk of fracture [2], which results in a marked increase in morbidity, and health care costs [34]. After the onset of menopause, increases in the incidence of osteoporosis result from a decrease in the circulating level of estrogen. Estrogen deficiency increases the life span as well as formation and activation of osteoclasts, thereby resulting in cortical porosity and enlarged resorption areas in trabecular surfaces [5]. Estrogen deficiency also induces apoptosis of osteoblasts which are involved in bone formation [6]. Therefore, although estrogen deficiency increases the bone turnover, there is an imbalance between bone formation and bone resorption.
Hormone replacement therapy (HRT) treats menopausal symptoms including decreases in bone mass. However, the benefits provided by HRT are often associated with increased risk of development of breast, ovarian and endometrial cancers [7]. In this regard, research efforts on phytoestrogens are being directed toward determining whether they act as estrogen antagonist in mammary and ovarian glands and, in contrast, as estrogen agonists in systems affected by menopause.
St. John's Wort (SJW: Hypericum perforatum) contains hypericin, hyperforin, and flavonoids. SJW has been used for the treatment of fibrosis, neuralgia, depression, and anxiety as an alternative to classic antidepressants [8910]. We previously demonstrated that SJW can prevent Ovariectomy (OVX)-induced obesity without stimulatory activity in the uterus [11]. However, there are few reports concerning the beneficial effects of SJW on osteoblast proliferation and trabecular bone loss induced by estrogen deficiency.
The following research was undertaken to characterize the preventive effect of SJW extract on MG-63 human osteoblast proliferation and trabecular bone loss in an ovariectomized rat model resembling the decline in estrogen levels in postmenopausal women.
Powdered SJW (500 g) was immersed in 5 L of 70% ethanol at 90℃ for 8~12 h, and this procedure was repeated in 2.5L of 70% ethanol at 90℃ for 8~12 h. The extract was concentrated under reduced pressure using a rotary evaporator (Daesin Machine Industry, Korea) at 60℃ and freeze-dried. The yield obtained was 16.3%.
MG-63 cells (Korean Cell Line Bank, Seoul, Korea) were cultured in Eagle Minimum Essential medium (MEM, Gibco Life Technologies, Grand Island, NY, USA) supplemented with 10% fetal bovine serum, 100 U/ml penicillin, and 100 U/ml streptomycin under a humidified 5% CO2 atmosphere at 37℃. The medium was renewed twice weekly, and the cells were subcultured at 3-day intervals, before reaching 80% confluence.
Cytotoxicity was evaluated using a Cell Counting Kit-8 (Dojindo, Kumamoto, Japan). MG-63 cells were seeded into wells of a 96-well microplate at a density of 2.0 × 104 cells/well and incubated at 37℃ for 24 h. After the incubation, the culture medium was replaced with 100 µl of MEM containing 0.2, 2, or 20 µg/ml SJW extract after which the cells were incubated for an additional 36 h. Thereafter, 10 µl of Cell Counting Kit-8 solution was added to each well, and the cells were incubated at 37℃ for 1 h. Cell cytotoxicity was assessed based on the optical density at 450 nm using VersaMax ELISA microplate reader (Molecular Devices, Sunnyvale, USA).
MG-63 cells were seeded into wells of a 96-well microplate at a density of 5 × 103 cells/well in normal growth medium. After 24 h, when the cells had attached to the well bottom, the medium was replaced with estrogen-free, phenol red-free MEM containing 5% charcoal-dextran-stripped fetal bovine serum. Different concentrations of SJW extract was added, and the cells were cultured for 192 h. A positive control was prepared by treating the cells with β-estradiol-3-benzoate (E2), while a negative control was prepared by using vehicle solvents. MG-63 cell proliferation was assessed based on the optical density at 450 nm using the aforementioned microplate reader.
MG-63 cells were seeded into wells of a 48-well plate at a density of 1 × 105 cells/well and incubated in growth medium for 24 h at 37℃ under a humidified 5% CO2 atmosphere. The medium was then replaced with estrogen-free, phenol red-free MEM containing 5% charcoal-dextran-stripped fetal bovine serum with 20 µg/mL of SJW extract. After incubating the cells for 36 h at 37℃, they were washed twice with PBS and total RNA was extracted using a RNeasy Plus Mini Kit (Qiagen, Carlsbad, USA) according to the manufacturer's protocol. Oligo-dT primers were then used to synthesize cDNA. The primer sequences for glyceraldehyde-3-phosphate dehydrogenase (GAPDH), alkaline phosphatase (ALP), osteocalcin (OC) and osteoprotegerin (OPG) and are listed in Table 1. For real-time PCR, reverse transcription was carried out for 1 h at 37℃, after which PCR was run using a SYBR Green Master kit (Takara, Japan). The PCR protocol entailed initial denaturation at 95℃ for 10 sec followed by 40 cycles of denaturation at 95℃ for 30 sec, annealing at 60℃ for 30 sec, and elongation at 72℃ for 30 sec. The relative changes in mRNA expression from the control were calculated using the 2ΔΔCt method. Ct values were normalized to those for GAPDH, and the ΔCt values of the treated cells were normalized to the mean ΔCt values of the controls.
For transient transfection, MG-63 cells were plated in wells of a 96-well microplate at a density of 2.0 × 104 cells/well. Once the cells reached 80% confluence, transfection was carried out using FuGENE®HD transfection reagent (Promega, Madison, USA) according to the manufacturer's protocol. Briefly, Opti-MEM® was used to dilute ER (estrogen receptor) α (pEGFP-C1-ER α, Addgene, Cambridge, USA), ER β (pcDNA Flag ER β, Addgene), and Renilla luciferase (pRL-SV40, Promega) prior to addition of the transfection reagent. The FuGENE® HD/plasmid ratio was 3:1. After a 10 min incubation at room temperature, 5 µl of FuGENE®HD complex mixture was added to the wells, and the cells were incubated for 24 h at 37℃ under a humidified 5% CO2 atmosphere.
For luciferase assays, transfected MG-63 cells were incubated for 36 h at 37℃ in phenol red-free MEM containing 5% charcoal-dextran stripped fetal bovine serum with 0.2, 2, or 20 µg/ml SJW extract. Following the incubation, the cells were washed twice with phosphate-buffered saline (PBS) and lysed in 20 µl of passive lysis buffer (Promega). Luciferase activity was then developed using Luciferase Assay Reagent (Promega) and quantified using a GloMax Multi Microplate Luminometer (Promega) according to the manufacturer's protocol. The luciferase activity was normalized to that of Renilla luciferase and expressed as relative luciferase activity (RLA) defined as a percentage of untreated negative control.
Female Sprague Dawley rats (9 weeks old) were purchased from Central Laboratory Animal (SLC. Inc., Japan). Animals were housed in a climate-controlled room (22 ± 2℃, 50 ± 10% relative humidity) under a 12 h light/dark cycle and provided diet and water ad libitum. The rats were acclimated for a week, and then divided into five groups: Sham control (Sham-C), ovariectomized control (OVX-C) and E2 or SJW extract (SJW100 or SJW 200) treated groups. The rats in OVX-C, E2 and SJW groups were ovariectomized and animals in Sham-C were sham operated and all animals were acclimated to diet for two more weeks. Two weeks after ovariectomy, we provided the animals with E2 at a dose of 50 µg/kg/day[five time a week, (E2)], and SJW at a dose of 100 (SJW100) or 200 (SJW200) mg/kg/day for six weeks and sacrificed. Animals in all groups were provided a modified AIN-93G control diet (7% corn oil replacing soybean oil). All experiments were approved by the guidelines of Laboratory Animal Care and Use Committee of Mokpo National University (MNU-IACUC-2014-006).
The weight and food intake of the animals were recorded every week. Serum alkaline phosphatase (Biovision, USA) and C-telopeptide (Mybiosource, USA) were measured using kits.
For tissue preparation and staining, bilateral femurs were fixed in 10% neutral buffered formalin, decalcified with 0.5M EDTA for 1 month, routinely processed, and embedded in paraffin wax. The sections were cut to 5 µm thickness and stained with hematoxylin and eosin (H&E).
Histomorphometric measurements involved the metaphyseal region of distal rat femur, also known as secondary spongiosa. All measurement was performed in 100× magnification with application Suite software version 2.8.1 (Leica Mycrosystem, Korea) followed by the proposed standardized system of the American Society for Bone and Mineral Research [12,13]. Trabecular bone volume (BV/TV), trabecular thickness (Tb.Th), trabecular number (Tb.N), and trabecular separation (Tb.Sp) were analyzed.
MG-63 human osteoblast cells were exposed to various concentrations of E2 or SJW, and intracellular toxicity was measured by MTT assay. SJW at all concentration showed no significant effect on viability after 24 h (Fig. 1). However, E2 was cytotoxic at 10-5 M. E2 increased cell proliferation at 10-9 and 10-7 M compared to the control group. At 10-5 M of E2, cell proliferation was not significantly different from the control group. SJW increased MG-63 cell proliferation (Fig. 2). E2 and SJW showed a biphasic effect on MG-63 cell proliferation over a concentration range of 10-9 and 10-5 M and 0.2 and 20 µg/ml, respectively.
We investigated the positive effect of SJW on gene expression of ALP, OC and OPG. SJW increased ALP gene expression to the similar level as that in E2 treated cells, positive control group. SJW increased the gene expression of OC and OPG compared to E2 (Fig. 3).
To determine the estrogenic effect of SJW, the expressions of ER α and ER β were determined. E2 showed increase in expression of ER. SJW also significantly increased expression of ER α and ER β at 20 µg/ml compared to control (Fig. 4). Compared to E2, SJW was more effective in increases of ER β expression than ER α expression.
Two weeks after ovariectomy, untreated OVX (OVX-C) rats showed significantly higher body weight than intact (Sham-C) rats (data are not shown). SJW significantly decreased body weight gains compared to OVX-C rats. However, the treatment dose did not produce a significant difference in body weight gain. The final body weight in rats treated with E2 was significantly lower than that of Sham-C. E2 significantly decreased food efficacy ratio, while SJW did not (Table 2).
Sham-C rats exhibited the most compact and thickest trabecular structure, whereas, OVX-C rats showed a few trabeculae in H&E staining of femoral metaphyseal region. The structure of the trabecular bone in rats given E2 was similar to that of sham-C. The rats given SJW displayed denser structure compared to rats in the OVX-C group, irrespective of the dose (Fig. 5). OVX reduced the bone volume/tissue volume (BV/TV) and trabecular number (Tb.N), and increased trabecular separation (Fig. 6). SJW at a dose of 200 mg/kg increased OVX-induced decreases in BV/TV and Tb.N, especially, BV/TV at the level of sham-C rats. SJW dose-dependently reduced OVX induced increase in Tb.Sp (Fig. 6).
ALP and CTX were measured as bone turnover makers in serum (Fig. 7). OVX resulted in a increase in serum ALP and CTX. SJW reduced ALP levels, although the reduction in serum ALP levels determined in OVX rats treated with SJW was not statistically significant. The CTX concentration in serum was significantly reduced by SJW.
The regulators of bone turnover are osteoblasts and osteoclasts, which are involved in bone formation and resorption, respectively. In the present study, we demonstrate that SJW extract increased MG-63 human osteoblast cell proliferation. We assumed that SJW increased differentiation of MG-63 cells by increasing the gene expression of ALP and OC, which are phenotypic markers for early and terminal stage of differentiation, respectively [14]. SJW also induced the gene expression of OPG. OPG plays a pivotal role in bone turnover via the OPG/RANK/RANKL [15]. RANKL, a transmembrane protein expressed by osteoblasts, binds to its receptor RANK on the surface of osteoclasts [1617]. Binding of RANKL to RANK activates a number of signaling pathways resulting in osteoclast formation, differentiation and activation, which stimulates bone resorption. OPG produced by osteoblasts binds to RANKL, preventing its binding to RANK and subsequent osteoclast activation [15]. Considering our results, SJW increased bone formation by stimulating osteoblast differentiation and proliferation, and reduced bone resorption by upregulating gene expression of OPG.
We determined the expression of ER α and ER β using a luciferase assay to verify the involvement of ER in MG-63 cell proliferation. The results of cell proliferation (Fig. 2) and ER expression (Fig. 4) revealed no correlation in MG-63 cell proliferation and ER expression. Although SJW at the concentration of 20 µg/ml increased the expression of ER α and ER β, the proliferation of MG-63 cells treated with 20 µg/ml of SJW was lower than that of MG-63 cells treated with 2 µg/ml of SJW. Previous researches [1819] reported that even though, estrogenicity of isoflavones for MG-63 cell proliferation is partially mediated by the ER system, other mechanisms including regulation of tyrosine kinase, activation of membrane-bound receptor signaling or cross-talk between the ER and growth factor signaling pathways could be involved. Considering this report and our result of cell proliferation, we can assume that MG-63 cell proliferation by SJW is not or partially mediated by ER system.
Since the consequences of estrogen deficiency are seen on trabecular rather than cortical bone [20], we examined the effect of SJW on trabecular structure in ovariectomized rats. The response to the estrogen deficiency is a dramatic increase in turnover of cancellous bone, consequently associated with an initial and rapid loss of trabecular structure [212223]. Bisphosphonate is an anti-catabolic agent that has been used as the mainstay therapy for osteoporosis and fracture prevention by improvement of trabecular bone mass [2024]. In the present study, histomorphometric analysis indicated that SJW at the concentration of 200 mg/kg could prevent OVX-induced trabecular bone loss. H&E staining also confirmed that SJW treated rats displayed denser structure in the metaphyseal region of distal femur compared to OVX-C rats. Serum concentration of ALP and CTX are bone turnover markers in mice [2526]. Shan et al. [27] reported that age-related change of bone turnover was reflected by serum ALP and CTX. High levels of serum ALP and CTX were significantly correlated with a low bone mineral density at the lumber spine, total hip and utradistal forearm regions. Additionally high CTX level may be a predictor for a low bone mineral density T-score in Chinese women. In the present study, OVX resulted in a significant increase in serum ALP and CTX, suggesting that OVX increased the bone turnover rate in these animals. Furthermore, SJW reduced OVX induced increases in ALP and CTX, and subsequent bone turnover rate. It is thus conceivable that SJW might be clinically useful and effective in the prevention for bone loss in postmenopausal women.
In conclusion, this study demonstrates the beneficial effects of SJW on MG-63 human osteoblast cell differentiation and proliferation, and also on skeletal tissues in an estrogen deficient animal model. In our previous study, we demonstrated that SJW acts as estrogen antagonist in uterine epithelial proliferation [11]. Therefore, the evidence clearly demonstrates that SJW can be considered as a natural alternative to hormone replacement therapy for the prevention and treatment of bone loss in postmenopausal women.
Figures and Tables
Fig. 1
Effect of St. John's Wort extract on cytotoxicity in MG 63 cells.
Values are mean ± SD. Stand error bars represent three independent experiments and each experiment was triplicated. Means with the same letter are not significantly different by Duncan's multiple range test (P < 0.05). SJW: 70% ethanol extract of St. John's Wort

Fig. 2
Effect of St. John's Wort extract on proliferation in MG 63 cells.
Values are mean ± SD. Stand error bars represent three independent experiments and each experiment was triplicated. Means with the same letter are not significantly different by Duncan's multiple range test (P < 0.05). SJW: 70% ethanol extract of St. John's Wort

Fig. 3
Effect of St. John's Wort extract on the gene expression of alkaline phosphatase (A), osteocalcin (B) and osteoprotegerin (C) in MG 63 cells.
Values are mean ± SD. Stand error bars represent three independent experiments and each experiment was triplicated. Means with the same letter are not significantly different by Duncan's multiple range test (P < 0.05). SJW: 70% ethanol extract of St. John's Wort

Fig. 4
Effect of St. John's Wort extract on ER α (A) and ER β (B) in MG 63 cells.
Values are mean ± SD. Stand error bars represent three independent experiments and each experiment was triplicated. Means with the same letter are not significantly different by Duncan's multiple range test (P < 0.05). SJW: 70% ethanol extract of St. John's Wort

Fig. 5
Histopathology of the metaphyseal region of distal rat femur in each group (H&E).
100× magnification, scale bar = 200 µm. Sham-C: Sham operation + distilled water, OVX-C: Ovariectomy + distilled water, E: Ovariectomy + estradiol 50 µg/kg body weight, SJW100: Ovariectomy + St. John's Wort extract 100 mg/kg body weight, SJW200: Ovariectomy + St. John's Wort extract 200 mg/kg body weight
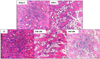
Fig. 6
Effect of St. John's Wort extract on Bone volume/tissue volume (A), Trabecular thickness (B), Trabecular number (C) and Trabecular separation (D) in ovariectomized rats.
Values are mean ± SD. Means with the same letter are not significantly different by Duncan's multiple range test (P < 0.05). Sham-C: Sham operation + distilled water, OVX-C: Ovariectomy + distilled water, E: Ovariectomy + estradiol 50 µg/kg body weight, SJW100: Ovariectomy + St. John's Wort extract 100 mg/kg body weight, SJW200: Ovariectomy + St. John's Wort extract 200 mg/kg body weight

Fig. 7
Effect of St. John's Wort extract on the serum alkaline phosphatase (A) and C-telopeptide (B)
Values are mean ± SD. Means with the same letter are not significantly different by Duncan's multiple range test (P < 0.05). Sham-C: Sham operation + distilled water, OVX-C: Ovariectomy + distilled water, E: Ovariectomy + estradiol 50 µg/kg body weight, SJW100: Ovariectomy + St. John's Wort extract 100 mg/kg body weight, SJW200: Ovariectomy + St. John's Wort extract 200 mg/kg body weight

Table 2
Effect of St. John's Wort extract on the body weight, food intake and food efficacy ratio

Values are mean ± SD. Means with the same letter in a column are not significantly different by Duncan's multiple range test (P < 0.05). Sham-C: Sham operation + distilled water, OVX-C: Ovariectomy + distilled water, E: Ovariectomy + estradiol 50 µg/kg body weight, SJW100: Ovariectomy + St. John's Wort extract 100 mg/kg body weight, SJW200: Ovariectomy + St. John's Wort extract 200 mg/kg body weight
References
1. Clowes JA, Riggs BL, Khosla S. The role of the immune system in the pathophysiology of osteoporosis. Immunol Rev. 2005; 208:207–227.

2. Marshall D, Johnell O, Wedel H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ. 1996; 312:1254–1259.

3. Osteoporosis . review of the evidence for prevention, diagnosis and treatment and cost-effective analysis. Osteoporos Int. 1998; 8:Suppl 4. S1–88.
4. Royal College of Physicians of London. Osteoporosis: Clinical Guidelines for Prevention and Treatment. London: Royal College of Physicians of London;1999.
6. Kousteni S, Han L, Chen JR, Almeida M, Plotkin LI, Bellido T, Manolagas SC. Kinase-mediated regulation of common transcription factors accounts for the bone-protective effects of sex steroids. J Clin Invest. 2003; 111:1651–1664.

7. Gallo D, Zannoni GF, Apollonio P, Martinelli E, Ferlini C, Passetti G, Riva A, Morazzoni P, Bombardelli E, Scambia G. Characterization of the pharmacologic profile of a standardized soy extract in the ovariectomized rat model of menopause: effects on bone, uterus, and lipid profile. Menopause. 2005; 12:589–600.

8. Mennini T, Gobbi M. The antidepressant mechanism of Hypericum perforatum. Life Sci. 2004; 75:1021–1027.

9. Brenner R, Azbel V, Madhusoodanan S, Pawlowska M. Comparison of an extract of hypericum (LI 160) and sertraline in the treatment of depression: a double-blind, randomized pilot study. Clin Ther. 2000; 22:411–419.

10. Schrader E. Equivalence of St John's wort extract (Ze 117) and fluoxetine: a randomized, controlled study in mild-moderate depression. Int Clin Psychopharmacol. 2000; 15:61–68.

11. You MK, Rhuy J, Jeong KS, Bang MA, Kim MS, Kim HA. Effect of St. John's Wort (Hypericum perforatum) on obesity, lipid metabolism and uterine epithelial proliferation in ovariectomized rats. Nutr Res Pract. 2014; 8:292–296.

12. Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR. Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 1987; 2:595–610.

13. Dempster DW, Compston JE, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR, Parfitt AM. Standardized nomenclature, symbols, and units for bone histomorphometry: a 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 2013; 28:2–17.

14. Cho YS, Jung WK, Kim JA, Choi IL, Kim SK. Beneficial effects of fucoidan on osteoblastic MG-63 cell differentiation. Food Chem. 2009; 116:990–994.

15. Reid P, Holen I. Pathophysiological roles of osteoprotegerin (OPG). Eur J Cell Biol. 2009; 88:1–17.

16. Udagawa N, Takahashi N, Yasuda H, Mizuno A, Itoh K, Ueno Y, Shinki T, Gillespie MT, Martin TJ, Higashio K, Suda T. Osteoprotegerin produced by osteoblasts is an important regulator in osteoclast development and function. Endocrinology. 2000; 141:3478–3484.

17. Dougall WC, Chaisson M. The RANK/RANKL/OPG triad in cancerinduced bone diseases. Cancer Metastasis Rev. 2006; 25:541–549.

18. Kim J, Um SJ, Woo J, Kim JY, Kim HA, Jang KH, Kang SA, Lim BO, Kang I, Choue RW, Cho Y. Comparative effect of seeds of Rhynchosia volubilis and soybean on MG-63 human osteoblastic cell proliferation and estrogenicity. Life Sci. 2005; 78:30–40.

19. Webb P, Nguyen P, Valentine C, Lopez GN, Kwok GR, McInerney E, Katzenellenbogen BS, Enmark E, Gustafsson JA, Nilsson S, Kushner PJ. The estrogen receptor enhances AP-1 activity by two distinct mechanisms with different requirements for receptor transactivation functions. Mol Endocrinol. 1999; 13:1672–1685.

20. Watkins MP, Norris JY, Grimston SK, Zhang X, Phipps RJ, Ebetino FH, Civitelli R. Bisphosphonates improve trabecular bone mass and normalize cortical thickness in ovariectomized, osteoblast connexin43 deficient mice. Bone. 2012; 51:787–794.

21. Hodgkinson A, Aaron JE, Horsman A, McLachlan MS, Nrodin BE. Effect of oophorectomy and calcium deprivation on bone mass in the rat. Clin Sci Mol Med. 1978; 54:439–446.

22. Wronski TJ, Lowry PL, Walsh CC, Ignaszewski LA. Skeletal alterations in ovariectomized rats. Calcif Tissue Int. 1985; 37:324–328.

23. Wronski TJ, Dann LM, Scott KS, Cintrón M. Long-term effects of ovariectomy and aging on the rat skeleton. Calcif Tissue Int. 1989; 45:360–366.

24. Faienza MF, Ventura A, Marzano F, Cavallo L. Postmenopausal osteoporosis: the role of immune system cells. Clin Dev Immunol. 2013; 2013:575936.

25. Srivastava AK, Bhattacharyya S, Castillo G, Miyakoshi N, Mohan S, Baylink DJ. Development and evaluation of C-telopeptide enzyme-linked immunoassay for measurement of bone resorption in mouse serum. Bone. 2000; 27:529–533.

26. Srivastava AK, Bhattacharyya S, Li X, Mohan S, Baylink DJ. Circadian and longitudinal variation of serum C-telopeptide, osteocalcin, and skeletal alkaline phosphatase in C3H/HeJ mice. Bone. 2001; 29:361–367.

27. Shan PF, Wu XP, Zhang H, Luo XH, Cao XZ, Xie H, Liu SP, Pi YZ, Fang TY, Liu H, Chen ZH, Zhong N, Liao EY. Age-related changes of serum bone alkaline phosphatase and cross-linked C-telopeptides of type I collagen and the relationship with bone mineral density in Chinese women. Clin Chim Acta. 2006; 366:233–238.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download