Abstract
BACKGROUND/OBJECTIVES
This study was conducted to investigate the effects of fermented soybean (FS) extract on adipocyte differentiation and fat accumulation using cultured 3T3-L1 adipocytes.
MATERIALS/METHODS
3T3-L1 adipocytes were treated with FS and nonfermented soybean (NFS) extract during differentiation for 10 days in vitro. Oil red O staining was performed and glycerol-3-phosphate dehydrogenase (GPDH) activity was measured for analysis of fat accumulation. Expressions of adipogenic genes were measured.
RESULTS
Soluble extract of soybean fermented with Aspergillus oryzae GB107 contained higher levels of low-molecular-weight protein than conventional soybean protein did. FS extract (50 µg/ml) inhibited adipocyte differentiation and fat accumulation during differentiation of 3T3-L1 preadipocytes for 10 days in vitro. Significantly lower GPDH activity was observed in differentiated adipocytes treated with the FS extract than those treated with NFS extract. Treatment with FS extract resulted in decreased expression levels of leptin, adiponectin, and adipogenin genes, which are associated with adipogenesis.
Soybean, a high-quality protein source, has been widely used in traditional nonfermented foods, such as soy milk, tofu, and tofu skin, and in fermented foods, such as miso, soy sauce, bean paste, natto, and tempeh. In a previous study, we demonstrated that solid-state fermentation of soybean by Aspergillus oryzae (A. oryzae) GB107 improves the nutritional quality of soybean food and feed meals [1]. The use of fermented soybean (FS) meal as a replacement for conventional soybean meal improved the efficiency of nutrient utilization and the diarrhea score of nursery pigs [2]. In addition, FS decreased the immune response by lipopolysaccharide in nursery pigs [3].
Obesity is characterized by excessive accumulation and storage of body fat and results in abnormalities in energy storage and utilization. This increase in adipose tissue mass occurs as a result of both hypertrophy and hyperplasia of adipocytes, the latter being due to increased differentiation of preadipocytes [456]. The discovery that adipose cells secrete leptin, which regulates food intake and energy homeostasis [7], confirmed the role of adipose tissue as a secretory organ. In addition to leptin, adipocytes have been found to secrete adipokines, i.e., adiponectin, tumor necrosis factor-α (TNF-α), chemerin, and interleukin-6 (IL-6) [8910111213]. The development of an in vitro preadipocyte culture system has facilitated the elucidation of the mechanism of adipogenesis and has shown that the key players in adipogenesis are peroxisome proliferator-activated receptor-γ2 (PPAR-γ2), CCAAT/enhancer binding protein (C/EBP)-α, C/EBP-β, and sterol regulatory element-binding protein-1c (SREBP-1c) [514].
Natural and synthetic agents that are effective in prevention of both fat- and sugar-induced obesity can exert anti-obesity effects by increasing lipolysis in white adipocytes and by blocking adipocyte differentiation. FS exhibits several biological functions depending on the microbe used for fermentation [15]. In this study, we investigated whether the soluble extract of soybean fermented with A. oryzae GB107 modulates fat accumulation and lipogenesis in cultured 3T3-L1 adipocytes. Our results show that the extract of soybean fermented with A. oryzae GB107 could be used to prevent obesity and obesity-induced diseases.
FS was prepared by a commercial company (Genebiotech Co. Ltd., Seoul, Korea) as previously reported [1]. Water-soluble proteins were extracted from FS and NFS using a modified method based on a previous report [1]. Ground samples (0.125 g) were homogenized for 5 minutes on ice with lysis buffer containing 4 mL of 20 mM Tris-HCl buffer (pH 7.6) including 0.1% sodium dodecyl sulfate, 5 mM dithiothreitol, and 5 µg/mL protease-inhibitor cocktail (Nacalai Tesque, Kyoto, Japan). Homogenized samples were centrifuged at 17,675 × g for 15 minutes at 4℃, and the supernatants were transferred to 1.5-mL microcentrifuge tubes and used for protein analysis. The protein content of each sample was determined using a Protein Assay Kit (Bio-Rad, Richmond, CA, USA). Proteins were subjected to SDS-PAGE using 5-12% gradient polyacrylamide gels. Coomassie blue-stained gels were scanned with a Bioimage system (BioImage, Ann Arbor, MI, USA). The remaining prepared supernatants were used for treatment of cultured adipocytes.
3T3-L1 preadipocytes were cultured and differentiated according to methods described in a previous report [16]. Briefly, the cells (5 × 104 cells/well) were plated and grown for 2 days post-confluence in six-well tissue culture plates in DMEM containing 10% fetal bovine serum, and the medium was changed every 48 hours. Cells were induced to differentiate by replacing the medium with serum-containing DMEM with 0.5 mM 3-isobutyl-1-methylxanthine (IBMX), 0.25 µM dexamethasone, and 1 µg/mL insulin. Two days later, the medium was again changed to serum-containing DMEM with insulin but no IBMX or dexamethasone. Two days later, the medium was again changed to DMEM containing 10% fetal bovine serum in the absence of any differentiating reagents, and it was replaced every 2 days from that day onward. For experimental purposes, the cells were treated and changed with differentiation medium with vehicle control, FS or NFS extracts (50 µg/ml) every 2 days during their differentiation. For controls, cells were treated with lysis buffer diluted with medium as vehicle. A preliminary dose-finding experiment concluded that a single dose (50 µg/ml) of FS and NFS extracts was suitable. The culture medium was collected every 2 days to examine glycerol release from 3T3-L1 adipocytes. After 10 days of differentiation, fat accumulation was determined by GPDH activity and oil red O staining. In addition, reverse transcription polymerase chain reaction (RT-PCR) was performed to monitor the expression levels of genes associated with adipogenesis.
3T3-L1 preadipocytes were cultured in a 96-well dish. After treatment with FS and NFS for 24 h, a 20 µl aliquot of 3-(4,5-Dimethylthiazol-2-yl)-2,5-dipheny ltetraoliumbromide (MTT, a yellow tetrazole; 5 mg/ml in PBS) was added to the wells, followed by incubation for 24 h at 37℃, as described in a previous report [17]. The supernatant was removed carefully, 200 µl of DMSO was added and mixed, and the absorbance was read at 563 nm.
Cytoplasmic lipid droplets were stained with oil red O, as described in a previous report [12]. Briefly, cells were rinsed three times in phosphate-buffered saline (PBS) and then fixed in 10% (v/v) formaldehyde for 10 min. The cells were washed twice with PBS, followed by staining for 30 min at 37℃ in freshly diluted oil red O (Sigma Chemical Co., St. Louis, MO, USA) solution (six parts oil red O stock and four parts H2O; oil red O stock solution is 0.5% oil red O in isopropanol), followed by further washing with PBS. The stained cytoplasmic triglycerides were visualized and photographed using a microscope. For quantification of oil red O content, the cells were washed 3 times with distilled H2O for removal of background staining, and isopropanol was added to resolve oil red O. The OD510 nm of the de-staining isopropanol was measured by spectrophotometry.
To monitor the differentiation of preadipocytes, the activity of the marker enzyme GPDH was analyzed using a commercial kit (GPDH activity measuring test; Hokudo, Sapporo, Japan), according to our previous report [18]. The protein content of each sample was determined using a Protein Assay Kit (Bio-Rad, Richmond, CA, USA).
Lipolytic activity was measured based on glycerol release from 3T3-L1 adipocytes over 2 days during treatment with FS or NFS extracts. Glycerol levels in the culture medium were analyzed using a Glyceride E-test Wako kit (Wako Pure Chemical Co. Ltd., Osaka, Japan).
Total RNA from 3T3-L1 cells was extracted using Trizol® reagent (Gibco/Invitrogen BRL, Rockville, MD). Semi-quantitative RT-PCR was performed as previously described [19] to determine the mRNA expression levels of leptin, adiponectin, and adipogenin. β-actin, a house-keeping gene, was used as the internal control.
Total RNA (1 µg) was incubated at 70℃ for 5 minutes. After heating, the RNA was chilled rapidly on ice. Then, it was reverse-transcribed for synthesis of cDNA in a 20 µl RT reaction containing ReverTra ACE® (Toyobo, Osaka, Japan) and oligo-dT primers. The mixtures were incubated at 30℃ for 10 minutes, 42℃ for 60 minutes, and 95℃ for 5 minutes. The RT products were used for subsequent PCR amplification with 20-35 cycles, which is the linear increasing phase of the PCR products.
The PCR components included the following: 10 µL of Go Taq® Green Master Mix (Go Taq® Green Master Mix, Promega, Madison, WI, USA), 1 µL of primer mixture (1 pmol/µL), 1 µL of cDNA sample, and 8 µL of nuclease-free water. The primer sequences, product sizes, number of amplification cycles, and annealing temperatures used for semi-quantitative RT-PCR are shown in Table 1. PCR products were resolved on a 1.5% agarose gel, and the DNA was visualized by ethidium bromide staining and analyzed using ImageJ 1.47. The mRNA levels of leptin, adiponectin, and adipogenin were normalized based on the mRNA level of β-actin.
The results are representative of at least three independent experiments and expressed as the mean ± SEM values of three or six wells in each experimental group. Differences in the means of each treatment were determined by Duncan test. The level of significance was uniformly set at P < 0.05.
The profiles of water-soluble proteins extracted from FS and NFS were examined and confirmed using SDS-PAGE gels. FS extracts contained higher levels of low-molecular-weight proteins (24-30 kDa) than NFS extracts (Fig. 1), and levels of low-molecular-weight proteins below 17 kDa were higher in FS extracts than in NFS extracts.
We performed an MTT assay to determine whether FS and NFS treatment affects cell viability in 3T3-L1 preadipocytes. The MTT assay revealed no changes in preadipocyte viability at all tested concentrations of FS and NFS (Fig. 2A and B).
To investigate the effect of FS and NFS extracts on 3T3-L1 preadipocyte differentiation, cells were treated with soybean extracts with a dose of 50 µg/ml. On microscopic examination, the FS extract-treated cells showed a low adipocyte number and a lower degree of adiposity compared with that for all other treatments (Fig. 3A, B). No changes were observed in adipocytes treated with lower doses of 50µg/ml. In addition, the lowest optical density values were obtained for adipocytes treated with FS extract (Fig. 3C).
GPDH occupies a central position in the triglyceride synthesis pathway, at the point where it branches from the glycolytic pathway [20]. Therefore, GPDH enzyme activity was measured in differentiated adipocytes treated with FS or NFS extracts. GPDH enzyme activity was significantly lower in differentiated adipocytes treated with the FS extract than in cells treated with the NFS extract (Fig. 4).
To determine the effects of FS and NFS extracts on lipolysis, glycerol release into cell culture medium was measured during adipocyte differentiation. After 8 days of adipocyte differentiations, treatment of FS extract group was enhanced glycerol release whereas treatment of NFS extract group was significantly blocked (Fig. 5).
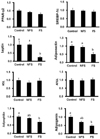
Significantly lower mRNA levels of leptin and adiponectin were observed in adipocytes treated with the FS extract compared to those treated with the control and NFS (Fig. 6). However, the levels of PPAR-γ2, SREBP-1c, HSL, and ATGL expression did not change among the treatments. The expression levels of adipophilin and adipogenin were lower than those of control and NFS.
To the best of our knowledge, this report is the first to demonstrate that the water-soluble extract from soybean fermented with A. oryzae GB107 inhibits fat accumulation and GPDH activity, stimulates lipolysis in adipocytes, and downregulates leptin and adiponectin gene expression. Fat accumulation and lipogenesis could be inhibited by one or multiple components extracted from FS. In comparison of FS and NFS extracts by protein electrophoresis, we observed that the FS extract consisted of more low-molecular-weight proteins (24-30 kDa and less than 17 kDa) than the NFS extract. Several types of enzymes break the large proteins in soybean to small molecules such as peptides and amino acids, which contribute the unique sensory and functional properties of the final products [15]. To date, many studies have reported on the anti-obesity and anti-lipogenic effects of soybean [17212223242526]. For example, black soybean anthocyanins have been reported to inhibit preadipocyte proliferation and lipid accumulation during differentiation and to reduce basal lipolysis in 3T3-L1 cells [18]. β-Conglycinin protein is a source of active peptides that inhibit fatty acid synthase activity and lipid accumulation in human adipocytes and 3T3-L1 adipocytes [2122]. Peptides from meju, long-term fermented soybeans, enhance the antidiabetic effect of soybeans in vitro [27]. Natto water-soluble fractions, comprising a low-molecular-weight viscous substance, and soybean water-soluble extract exert inhibitory effects on the oxidation of low-density lipoproteins, prevent arteriosclerosis, reduce lipid peroxidation, and improve lipid metabolism in vivo and in vitro [2829]. Soybean saponin improves cholesterol metabolism by stimulating the excretion of bile acid [23242526]. Lunasin has been shown to have the biologic properties of anti-inflammatory activities [30]. In this study, the active components in the FS extract responsible for the inhibitory effect on fat accumulation and lipogenesis were not identified; therefore, further studies are needed in order to characterize the components with anti-lipogenic activity.
Expression of adiponectin and leptin genes increases during adipocyte differentiation and fat accumulation. A decrease in adiponectin and leptin mRNA levels was observed in adipocytes treated with the FS extract, while adipophilin and adipogenin mRNA levels decreased. However, the expression of PPAR-γ2 and SREBP-1c, which are transcription factors involved in adipocyte differentiation, did not differ between NFS and FS extract-treated adipocytes. In this study, the expression levels of adipogenic genes were analyzed in cultured cells that were continuously treated for 10 days. This result suggests that NFS treatment did not affect the degree of adipocyte differentiation. Furthermore, these results suggest the possibility that fat accumulation and lipid storage are inhibited by the proteins in FS. However, no significant difference in the mRNA levels of HSL and ATGL, targeted genes of PPAR-γ2, were observed between groups In addition, no difference in mRNA levels of SREBP-1c and its downregulated gene ACC were observed for the NFS and FS extracts (ACC data was not shown). The inhibition of fat accumulation that we observed in this study involved several processes: (1) reduction in the activity of GPDH, an enzyme involved in fat synthesis; (2) reduced transcription of adipogenin mRNA, associated with fat cell differentiation; (3) reduced transcription of adipophilin mRNA, associated with formation of the fat droplet membrane; and (4) increased glycerol release from adipocytes in culture medium.
In conclusion, the water-soluble protein extracted from soybean fermented with A. oryzae GB107 inhibited fat accumulation and lipid storage in 3T3-L1 adipocytes. Therefore, FS components could be important food ingredients for control of lipid accumulation in adipose tissue.
Figures and Tables
Fig. 1
Distribution of the water-soluble proteins extracted from FS and NFS. Approximately 25 µg of protein was loaded per lane.
Lanes M contain Marker.
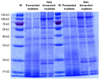
Fig. 2
Effect of (A) FS and (B) NFS on the viability of 3T3L-1 preadipocytes by MTT assay.
Values are expressed as a percentage survival compared to control (0 µg/ml) after 24 h incubation.

Fig. 3
Morphological changes in 3T3-L1 cells cultured with NFS extract (50 µg/ml) or FS extract (50 µg/ml).
(A) After 10 days, the cells were fixed and stained with oil red O for detection of oil droplets. (B) Magnified photographs of 3T3-L1 adipocytes cultured with NFS extract (50 µg/ml) or FS extract (50 µg/ml). (C) The stained cells were destained with isopropanol, and the OD of the de-staining isopropanol was measured by spectrophotometry. Values are expressed as mean ± SEM (n = 6). abMean values with different superscripts are significantly different (P < 0.05).
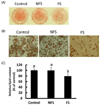
Fig. 4
GPDH activity of 3T3-L1 adipocytes cultured with NFS (50 µg/ml) or FS extract (50 µg/ml) for 10 days. Values are expressed as mean ± SEM (n = 6).
abMean values with different superscripts are significantly different (P < 0.05).

Fig. 5
Glycerol release from 3T3-L1 adipocytes on days 0, 2, 4, 6, 8, and 10 after induction of adipocyte differentiation.
3T3-L1 cells were cultured with NFS extract (50 µg/ml) or FS extract (50 µg/ml) after induction of differentiation. Data were normalized with respect to 100% for the control at day 0. Values are expressed as mean ± SEM (n = 3). abMean values with different superscripts are significantly different (P < 0.05).

Fig. 6
Expression levels of adipogenic-associated genes in 3T3-L1 adipocytes cultured with NFS (50 µg/ml) or FS (50 µg/ml) extracts for 10 days.
The data were normalized to β-actin mRNA levels, and values are expressed as the fold-value of that obtained for the control. Values are expressed as mean ± SEM (n = 3). abMean values with different superscripts are significantly different (P < 0.05).
References
1. Hong KJ, Lee CH, Kim SW. Aspergillus oryzae GB-107 fermentation improves nutritional quality of food soybeans and feed soybean meals. J Med Food. 2004; 7:430–435.

2. Kim SW, van Heugten E, Ji F, Lee CH, Mateo RD. Fermented soybean meal as a vegetable protein source for nursery pigs: I. Effects on growth performance of nursery pigs. J Anim Sci. 2010; 88:214–224.

3. Roh SG, Carroll JA, Kim SW. Effects of fermented soybean meal on innate immunity-related gene expressions in nursery pigs acutely challenged with lipopolysaccharides. Anim Sci J. Forthcoming 2014.

4. Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998; 395:763–770.

5. Gregoire FM, Smas CM, Sul HS. Understanding adipocyte differentiation. Physiol Rev. 1998; 78:783–809.

6. Roh SG, Hishikawa D, Hong YH, Sasaki S. Control of adipogenesis in ruminants. Anim Sci J. 2006; 77:472–477.

7. Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994; 372:425–432.

8. Sethi JK, Xu H, Uysal KT, Wiesbrock SM, Scheja L, Hotamisligil GS. Characterisation of receptor-specific TNFalpha functions in adipocyte cell lines lacking type 1 and 2 TNF receptors. FEBS Lett. 2000; 469:77–82.

9. Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, Sugiyama T, Miyagishi M, Hara K, Tsunoda M, Murakami K, Ohteki T, Uchida S, Takekawa S, Waki H, Tsuno NH, Shibata Y, Terauchi Y, Froguel P, Tobe K, Koyasu S, Taira K, Kitamura T, Shimizu T, Nagai R, Kadowaki T. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003; 423:762–769.

10. Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993; 259:87–91.

11. Roh SG, Song SH, Choi KC, Katoh K, Wittamer V, Parmentier M, Sasaki S. Chemerin--a new adipokine that modulates adipogenesis via its own receptor. Biochem Biophys Res Commun. 2007; 362:1013–1018.

12. Suzuki Y, Hong YH, Song SH, Ardiyanti A, Kato D, So KH, Katoh K, Roh SG. The regulation of chemerin and CMKLR1 genes expression by TNF-α, adiponectin, and chemerin analog in bovine differentiated adipocytes. Asian-Australas J Anim Sci. 2012; 25:1316–1321.

13. Suzuki Y, Song SH, Sato K, So KH, Ardiyanti A, Kitayama S, Hong YH, Lee SD, Choi KC, Hagino A, Katoh K, Roh SG. Chemerin analog regulates energy metabolism in sheep. Anim Sci J. 2012; 83:263–267.

14. Tontonoz P, Hu E, Spiegelman BM. Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a lipid-activated transcription factor. Cell. 1994; 79:1147–1156.

15. Kwon DY, Daily JW 3rd, Kim HJ, Park S. Antidiabetic effects of fermented soybean products on type 2 diabetes. Nutr Res. 2010; 30:1–13.

16. Hong YH, Hishikawa D, Miyahara H, Tsuzuki H, Nishimura Y, Gotoh C, Choi KC, Hokari Y, Takagi Y, Lee HG, Cho KK, Roh SG, Sasaki S. Up-regulation of adipogenin, an adipocyte plasma transmembrane protein, during adipogenesis. Mol Cell Biochem. 2005; 276:133–141.

17. Kim HK, Kim JN, Han SN, Nam JH, Na HN, Ha TJ. Black soybean anthocyanins inhibit adipocyte differentiation in 3T3-L1 cells. Nutr Res. 2012; 32:770–777.

18. Choi KC, Shrestha YB, Roh SG, Hishikawa D, Kuno M, Tsuzuki H, Hong YH, Sasaki S. The role of phosphatidylinositol 3-kinase and mitogenic activated protein kinase on the differentiation of ovine preadipocytes. Asian-Australas J Anim Sci. 2003; 16:1199–1204.

19. Hong YH, Nishimura Y, Hishikawa D, Tsuzuki H, Miyahara H, Gotoh C, Choi KC, Feng DD, Chen C, Lee HG, Katoh K, Roh SG, Sasaki S. Acetate and propionate short chain fatty acids stimulate adipogenesis via GPCR43. Endocrinology. 2005; 146:5092–5099.

20. Wise LS, Green H. Participation of one isozyme of cytosolic glycerophosphate dehydrogenase in the adipose conversion of 3T3 cells. J Biol Chem. 1979; 254:273–275.

21. Gonzalez de Mejia E, Martinez-Villaluenga C, Roman M, Bringe NA. Fatty acid synthase and in vitro adipogenic response of human adipocytes inhibited by α and α' subunits of soybean β-conglycinin hydrolysates. Food Chem. 2010; 119:1571–1577.

22. Martinez-Villaluenga C, Bringe NA, Berhow MA, Gonzalez de Mejia E. β-Conglycinin embeds active peptides that inhibit lipid accumulation in 3T3-L1 adipocytes in vitro. J Agric Food Chem. 2008; 56:10533–10543.

23. Lovati MR, Manzoni C, Canavesi A, Sirtori M, Vaccarino V, Marchi M, Gaddi G, Sirtori CR. Soybean protein diet increases low density lipoprotein receptor activity in mononuclear cells from hypercholesterolemic patients. J Clin Invest. 1987; 80:1498–1502.

25. Sidhu GS, Oakenfull DG. A mechanism for the hypocholesterolaemic activity of saponins. Br J Nutr. 1986; 55:643–649.

26. Sugano M, Goto S, Yamada Y, Yoshida K, Hashimoto Y, Matsuo T, Kimoto M. Cholesterol-lowering activity of various undigested fractions of soybean protein in rats. J Nutr. 1990; 120:977–985.

27. Kwon DY, Hong SM, Ahn IS, Kim MJ, Yang HJ, Park S. Isoflavonoids and peptides from meju, long-term fermented soybeans, increase insulin sensitivity and exert insulinotropic effects in vitro. Nutrition. 2011; 27:244–252.

28. Iwai K, Nakaya N, Kawasaki Y, Matsue H. Inhibitory effect of natto, a kind of fermented soybeans, on LDL oxidation in vitro. J Agric Food Chem. 2002; 50:3592–3596.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download