1. Frazier TH, Stocker AM, Kershner NA, Marsano LS, McClain CJ. Treatment of alcoholic liver disease. Therap Adv Gastroenterol. 2011; 4:63–81.

2. Li Y, Wang S, Ni HM, Huang H, Ding WX. Autophagy in alcohol-induced multiorgan injury: mechanisms and potential therapeutic targets. Biomed Res Int. 2014; 2014:498491.

3. Lu Y, Cederbaum AI. CYP2E1 and oxidative liver injury by alcohol. Free Radic Biol Med. 2008; 44:723–738.

4. Albano E. Alcohol, oxidative stress and free radical damage. Proc Nutr Soc. 2006; 65:278–290.

5. Sakaguchi S, Takahashi S, Sasaki T, Kumagai T, Nagata K. Progression of alcoholic and non-alcoholic steatohepatitis: common metabolic aspects of innate immune system and oxidative stress. Drug Metab Pharmacokinet. 2011; 26:30–46.

6. Szabo G, Bala S. Alcoholic liver disease and the gut-liver axis. World J Gastroenterol. 2010; 16:1321–1329.

7. Tome S, Lucey MR. Review article: current management of alcoholic liver disease. Aliment Pharmacol Ther. 2004; 19:707–714.
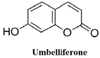
8. Kanimozhi G, Prasad NR, Ramachandran S, Pugalendi KV. Umbelliferone modulates gamma-radiation induced reactive oxygen species generation and subsequent oxidative damage in human blood lymphocytes. Eur J Pharmacol. 2011; 672:20–29.

9. Jung HA, Park JJ, Islam MN, Jin SE, Min BS, Lee JH, Sohn HS, Choi JS. Inhibitory activity of coumarins from Artemisia capillaris against advanced glycation endproduct formation. Arch Pharm Res. 2012; 35:1021–1035.

10. Zhao D, Islam MN, Ahn BR, Jung HA, Kim BW, Choi JS. In vitro antioxidant and anti-inflammatory activities of Angelica decursiva. Arch Pharm Res. 2012; 35:179–192.

11. Ramesh B, Pugalendi KV. Antihyperglycemic effect of umbelliferone in streptozotocin-diabetic rats. J Med Food. 2006; 9:562–566.

12. de Lima FO, Nonato FR, Couto RD, Barbosa Filho JM, Nunes XP, Ribeiro dos Santos R, Soares MB, Villarreal CF. Mechanisms involved in the antinociceptive effects of 7-hydroxycoumarin. J Nat Prod. 2011; 74:596–602.

13. Kassim NK, Rahmani M, Ismail A, Sukari MA, Ee GC, Nasir NM, Awang K. Antioxidant activity-guided separation of coumarins and lignan from Melicope glabra (Rutaceae). Food Chem. 2013; 139:87–92.

14. Ramesh B, Pugalendi KV. Antioxidant role of Umbelliferone in STZ-diabetic rats. Life Sci. 2006; 79:306–310.

15. Sim MO, Ham JR, Lee HI, Seo KI, Lee MK. Long-term supplementation of umbelliferone and 4-methylumbelliferone alleviates high-fat diet induced hypertriglyceridemia and hyperglycemia in mice. Chem Biol Interact. 2014; 216:9–16.

16. Vasconcelos JF, Teixeira MM, Barbosa-Filho JM, Agra MF, Nunes XP, Giulietti AM, Ribeiro-Dos-Santos R, Soares MB. Effects of umbelliferone in a murine model of allergic airway inflammation. Eur J Pharmacol. 2009; 609:126–131.

17. Lieber CS, DeCarli LM. Liquid diet technique of ethanol administration: 1989 update. Alcohol Alcohol. 1989; 24:197–211.
18. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001; 25:402–408.

19. Seo KI, Choi MS, Jung UJ, Kim HJ, Yeo J, Jeon SM, Lee MK. Effect of curcumin supplementation on blood glucose, plasma insulin, and glucose homeostasis related enzyme activities in diabetic db/db mice. Mol Nutr Food Res. 2008; 52:995–1004.

20. Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974; 249:7130–7139.
21. Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959; 82:70–77.

22. Wolff SP. [18] Ferrous ion oxidation in presence of ferric ion indicator xylenol orange for measurement of hydroperoxides. Methods Enzymol. 1994; 233:182–189.

23. Park WH, Park SY, Kim HM, Kim CH. Effect of a Korean traditional formulation, Hwaotang, on superoxide generation in human neutrophils, platelet aggregation in human blood, and nitric oxide, prostaglandin E2 production and paw oedema induced by carrageenan in mice. Immunopharmacol Immunotoxicol. 2004; 26:53–73.

24. Qin F, Sun HX. Immunosuppressive activity of Pollen Typhae ethanol extract on the immune responses in mice. J Ethnopharmacol. 2005; 102:424–429.

25. Mohamed MR, Emam MA, Hassan NS, Mogadem AI. Umbelliferone and daphnetin ameliorate carbon tetrachloride-induced hepatotoxicity in rats via nuclear factor erythroid 2-related factor 2-mediated heme oxygenase-1 expression. Environ Toxicol Pharmacol. 2014; 38:531–541.

26. Tobias PS, Soldau K, Gegner JA, Mintz D, Ulevitch RJ. Lipopolysaccharide binding protein-mediated complexation of lipopolysaccharide with soluble CD14. J Biol Chem. 1995; 270:10482–10488.

27. Yoon SJ, Koh EJ, Kim CS, Zee OP, Kwak JH, Jeong WJ, Kim JH, Lee SM. Agrimonia eupatoria protects against chronic ethanol-induced liver injury in rats. Food Chem Toxicol. 2012; 50:2335–2341.

28. Tilg H, Diehl AM. Cytokines in alcoholic and nonalcoholic steatohepatitis. N Engl J Med. 2000; 343:1467–1476.

29. Aono K, Isobe K, Kiuchi K, Fan ZH, Ito M, Takeuchi A, Miyachi M, Nakashima I, Nimura Y. In vitro and in vivo expression of inducible nitric oxide synthase during experimental endotoxemia: involvement of other cytokines. J Cell Biochem. 1997; 65:349–358.

30. Luster MI, Germolec DR, Yoshida T, Kayama F, Thompson M. Endotoxin-induced cytokine gene expression and excretion in the liver. Hepatology. 1994; 19:480–488.

31. An L, Wang X, Cederbaum AI. Cytokines in alcoholic liver disease. Arch Toxicol. 2012; 86:1337–1348.

32. Lee SH, Lee E, Ko YT. Anti-inflammatory effects of a methanol extract from Pulsatilla koreana in lipopolysaccharide-exposed rats. BMB Rep. 2012; 45:371–376.

33. Thompson KC, Trowern A, Fowell A, Marathe M, Haycock C, Arthur MJ, Sheron N. Primary rat and mouse hepatic stellate cells express the macrophage inhibitor cytokine interleukin-10 during the course of activation In vitro. Hepatology. 1998; 28:1518–1524.

34. Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001; 19:683–765.

35. Hill DB, Marsano L, Cohen D, Allen J, Shedlofsky S, McClain CJ. Increased plasma interleukin-6 concentrations in alcoholic hepatitis. J Lab Clin Med. 1992; 119:547–552.
36. Zima T, Kalousová M. Oxidative stress and signal transduction pathways in alcoholic liver disease. Alcohol Clin Exp Res. 2005; 29:110S–115S.

37. Suhail M, Suhail S, Gupta BK, Bharat V. Malondialdehyde and antioxidant enzymes in maternal and cord blood, and their correlation in normotensive and preeclamptic women. J Clin Med Res. 2009; 1:150–157.

38. Zhu H, Jia Z, Misra H, Li YR. Oxidative stress and redox signaling mechanisms of alcoholic liver disease: updated experimental and clinical evidence. J Dig Dis. 2012; 13:133–142.

39. Mohd Yusof H, Ali NM, Yeap SK, Ho WY, Beh BK, Koh SP, Long K, Abdul Aziz S, Alitheen NB. Hepatoprotective effect of fermented soybean (Nutrient Enriched Soybean Tempeh) against alcohol-induced liver damage in mice. Evid Based Complement Alternat Med. 2013; 2013:274274.

40. Park HY, Ha SK, Eom H, Choi I. Narirutin fraction from citrus peels attenuates alcoholic liver disease in mice. Food Chem Toxicol. 2013; 55:637–644.

41. Niemelä O, Alatalo P. Biomarkers of alcohol consumption and related liver disease. Scand J Clin Lab Invest. 2010; 70:305–312.

42. Kim MJ, Sim MO, Lee HI, Ham JR, Seo KI, Lee MK. Dietary umbelliferone attenuates alcohol-induced fatty liver via regulation of PPARα and SREBP-1c in rats. Alcohol. 2014; 48:707–715.








 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download