Abstract
BACKGROUND/OBJECTIVES
Yacon (Samallanthus sonchifolius), a common edible plant grown throughout the world, is well known for its antidiabetic properties. It is also known to have several other pharmacological properties including anti-inflammatory, anti-oxidant, anti-allergic, and anti-cancer effects. To date, the effect of yacon on gliomas has not been studied. In this study, we investigated the effects of yacon on the migration and proliferation of C6 glioma cells stimulated by fetal bovine serum (FBS).
MATERIALS/METHODS
Cell growth and proliferation were determined by evaluating cell viability using an EZ-Cytox Cell Viability Assay Kit. FBS-induced migration of C6 glioma cells was evaluated by performing the scratch wound healing assay and the Boyden chamber assay. We also used western blot analysis to determine the expression levels of extracellular signal-regulated kinase 1/2 (ERK1/2), a major regulator of migration and proliferation of glioma cells. Matrix metallopeptidase (MMP) 9 and TIMP-1 levels were measured by performing reverse transcription PCR.
RESULTS
Yacon (300 µg/mL) reduced both the FBS-induced proliferation of C6 glioma cells and the dose-dependent migration of the FBS-stimulated C6 cells. FBS-stimulated C6 glioma cells treated with yacon (200 and 300 µg/mL) showed reduced phosphorylation of ERK1/2 and inhibition of MMP 9 expression compared to those shown by the untreated FBS-stimulated C6 cells. In contrast, yacon (200 and 300 µg/mL) induced TIMP-1 expression.
Gliomas have a very high mortality rate owing to a peculiar feature of the tumor-rapid cell migration-that cannot be controlled either by surgery or radio-irradiation. Therefore, glioma patients generally only survive for an average of one year from the time of tumor development. Gliomas are not only difficult to diagnose but are also difficult to treat, primarily because their pathology and pattern of invasion are still poorly understood [1,2,3].
Cell invasion coupled with increased rates of cell proliferation, neoangiogenesis, and extensive infiltration of brain parenchyma are characteristic features of progression of brain cancer [4,5]. The mitogen-activated protein kinase (MAPK) cell-signaling pathway is made up of three major proteins: p38 MAPK, Jun N-terminal kinase, and extracellular signal-regulated kinase 1/2 (ERK1/2). These have been reported to be the active molecules involved in the control of cell proliferation and migration in a diverse range of cell types. [6]. Specifically, ERK1/2 has been implicated in cell migration and proliferation in most cancer cells [7]. Matrix metalloproteinases (MMP) are extracellular endopeptidases that have been associated with tumor progression owing to their involvement in the destruction of the basal membranes of cells [8]. Hence, activation of MMP9 leads to high motility and an increased invasion capacity in cancerous cells. Elevated expression of the tissue inhibitor of metalloproteinases-1 (TIMP-1) can inhibit the expression of MMP9 [9,10,11].
Samallanthus sonchifolius, commonly known as yacon, is a foodstuff that promotes human health. Recently, several studies have suggested that a number of important functional foods are potential candidates as medicinal plants and herbs in traditional Korean Medicine due to their pharmacological and biological properties. Yacon consists of diverse bioactive compounds that do not only induce physical health effects but also promote physiological responses. Yacon contains phytoalexins for antimicrobial activity, chlorogenic acids as antioxidant, anti-inflammation, anti-allergic, anti-cancer, and probiotic properties that counteract obesity and diabetes [12,13,14,18,19,20]. Although, it has been widely reported that yacon exhibits an anti-cancer effect, its therapeutic effect on gliomas remains to be elucidated. In the present study, we demonstrate that yacon extract inhibits cell migration by down-regulating the expression of MMP9 and decreasing the phosphorylation of ERK1/2. These findings provide a mechanistic insight into how yacon extract suppresses glioma cell growth and progression.
Cell culture materials were purchased from Gibco BRL (Gaithersburg, USA). The EZ-Cytox Cell Viability Assay Kit was purchased from Daeil Lab Service (Seoul, Korea). GAPDH, ERK 1/2, and phospho-ERK1/2 antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, USA). All other chemicals were purchased from Sigma corporation.
One-hundred grams of the plant were blended, and the crude powder was extracted in 1000 mL of sterile deionized water at 100℃ for 3 h. The aqueous extract was concentrated up to 300 mL at 60℃ under vacuum by the rotary evaporator. The remaining debris was precipitated with 700 mL absolute ethanol at 4℃ for 3 days. The supernatant was discarded and then was concentrated at 60℃ under vacuum by the rotary evaporator. The concentrated extract was dissolved in 50 mL of sterile deionized water and then lyophilized by freeze-drying at -60℃.
Rat C6 glioma cells were obtained from the Korean Cell Line Bank (Seoul, Korea) and were cultured in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin at 37℃ in a 5% CO2 atmosphere. C6 glioma cells were seeded at 5 × 104 cells/mL in a 96-well microplate containing DMEM and were then incubated for 24 h. The cells were incubated with different concentrations of yacon extract (100, 200, and 300 µg/mL) in either FBS or FBS-deficient media for 24 h. Cell viability was determined using an EZ-Cytox Cell Viability Assay Kit. The cell viability was determined as a percentage by comparing the absorbance at 450 nm for the treated cells and untreated control cells that had been incubated in FBS-deficient media.
C6 glioma cells were seeded at a density of 1 × 105 cells/mL in six-well plates and incubated in an FBS-containing medium for 24 h. The medium was then replaced with a serum-free medium. Following serum starvation for 24 h, a scratch wound was made across the center of the monolayer of cells in each well by using a sterile 200-µL pipette tip. This was followed by incubation with or without yacon extract in a serum-containing medium for an additional 24 h. Images of the cells that had migrated into the cell-free scratch wound area were acquired using an inverted microscope (IX71; Olympus, Tokyo, Japan) fitted with a charge-coupled device (CCD) camera. The migration distance was measured using the ImageJ software package. The scratch wound areas were determined by the relative percentage compared to untreated control cells in FBS-deficient media.
Boyden chamber assay was performed as previously reported [15]. To determine the effect of yacon extract on the migration of C6 glioma cells, the Boyden chamber assay was performed in a 48-well chemotaxis chamber (Neuro-Probe, Gaithersburg, MD, USA). Briefly, polycarbonate filter membranes (Neuro-Probe) with a pore size of 8 µm were coated with 0.1% collagen type-I and dried at room temperature for 1 h. Two different concentrations of yacon extract (200 and 300 µg/mL in 10% FBS media) were prepared and then 27.8-µL portions were dispensed into the lower chamber of the wells. The lower chamber containing the extract was then covered by the coated membrane, and 50 µL of C6 glioma cells (in serum-free media) were loaded into the upper chamber at a concentration of 1 × 106 cells/mL. Following culture at 37℃ in 5% CO2, cells on the lower surface of the membrane were fixed with methanol and stained using Diff-Quick. After staining, cells that had migrated through the filter membrane were imaged and counted using an inverted microscope as described above.
To analyze protein expression, we performed western blotting using specific antibodies. Briefly, 20 µg of protein was prepared for each treatment group. After boiling, the proteins were separated by electrophoresis on 12% acrylamide gels and then transferred onto polyvinylidene difluoride membranes in transfer buffer at 4℃ for 2 h. The membrane was blocked with 5% BSA in Tris-buffered saline (TBS) at room temperature for 1 h and then washed in TBS with 0.1% Tween 20 (TBS/T). The membranes were incubated overnight at 4℃ with either phosphorylated ERK1/2 (p-ERK1/2) antibody, or ERK1/2 antibody, both at a 1 : 2000 dilution. The membranes were washed with TBS/T, followed by incubation with a 1 : 5000 dilution of IgG secondary antibody conjugated to horseradish peroxidase. The expression levels of the proteins were analyzed using chemiluminescence (ECL plus kit; Amersham Pharmacia Biotech). Developed protein bands were visualized and quantified using Image J Software.
Total RNA was isolated from cells using TRI-reagent and then cDNA was synthesized using 0.5 µg of total RNA; the superscript II reverse transcription system with oligo-deoxythymidine. PCR amplification was performed using the following protocol: a predenaturation at 95℃ for 3 min and then either 35 cycles of denaturation at 94℃ for 35 s, annealing at 52℃ for 35 s, and extension at 72℃ for 35 s, followed by a final extension at 72℃ for 10 min. The mRNA expression was quantified using an ethidium bromide-stained 1.5% agarose gel. The following primers were used for RT-PCR: MMP9, sense primer, 5'-AAGTTTGGTGTCGCGGAGCAC-3', and anti-sense primer, 5'-TACATGAGCGCTTCCGGCAC-3'; TIMP-1, sense primer, 5'-ACAGCTTTCTGCAACTCG-3', and anti-sense primer, 5'-CTATAGGTCTTTACGAAGGCC-3'; GAPDH, sense primer, 5'-GGCATTGCTCCTCAATGACAA-3', and anti-sense primer, 5'-TGTGAGGGAGATGCTCAGTG-3'.
The results are expressed as the mean ± standard error (SE) of at least three independent experiments. The difference between the two groups was examined using Student's t-test, and one-way analysis of variance (ANOVA) with Tukey's test was used for multiple comparisons (Prism 4.00 for Windows, GraphPad, San Diego, USA). A P-value of < 0.05 was considered as statistically significant.
To determine the effect of yacon extract on the viability of FBS-stimulated C6 glioma cells, we performed a XTT cell viability assay. To determine the effect of yacon extract on the FBS-stimulated cell growth of glioma cells, C6 glioma cells were treated with yacon extract (100, 200 and 300 µg/mL) in either FBS-deficient media or in 10% FBS for 24 h. As shown in Fig. 1, the cell viability was unaffected by yacon extract up to a concentration of 300 µg/ml. The percentage cell growth was 146 ± 2.6% in cells stimulated by 10% FBS. The FBS-induced C6 cell growth was reduced by 40.7 ± 4.1% in response to 300 µg/mL of yacon extract.
The scratch wound healing assay is commonly used as a method to test the migration rate for a variety of cell types. In this study, we carried out the scratch wound healing assay, to test the effect of yacon extract on the cell migration rate of FBS-stimulated C6 glioma cells. As shown in Fig. 2, the results showed a significant increase in cell motility of up to 222.4 ± 10% when cells were post-treated with 10% FBS in comparison to untreated cells. On the other hand, the FBS-stimulated C6 cells exhibited a reduction of 50.8 ± 15.8% and 88.5 ± 12.4% in the migration rate when treated with yacon extract at concentrations of 200 µg/mL and 300 µg/mL, respectively.
We performed the Boyden chamber assay to confirm the inhibitory effect of yacon extract on cell migration in FBS-stimulated C6 glioma cells. As shown in Fig. 3, the presence of 10% FBS significantly increased cell migration by 393.1 ± 12.3%. Treatment with 200 µg/mL and 300 µg/mL yacon extract reduced FBS-induced C6 cell migration by 222.0 ± 8.3% and 372.5 ± 12.7%, respectively.
Western blot assays were performed to analyze the degree of phosphorylation of ERK1/2 in FBS-stimulated C6 glioma cells. As shown in Fig. 4, the expression of the phosphorylated ERK1/2 was increased by 75.33 ± 6.8% in cells stimulated by 10% FBS, compared to those not treated with FBS or exposed to yacon extract. In addition, yacon extract significantly reduced the phosphorylation of ERK1/2 by 33.0 ± 1.7% and 69.52 ± 5.2% at concentrations of 200 µg/mL and 300 µg/mL of yacon extract respectively.
Cell migration plays an important role in glioma cell progression, a phenomenon that is associated with increased MMP9. In contrast, the MMP9 activity can be controlled by the TIMP-1. Therefore, we employed reverse transcription PCR using specific primers to determine whether yacon extract could affect the mRNA expression levels of MMP9 and TIMP-1 in FBS-stimulated C6 glioma cells. As shown in Fig. 5, the elevation of MMP9 expression was enhanced by 10% FBS resulting in an increase of 75.15 ± 5.0%. Yacon extract significantly reduced the expression of MMP9 by 32.0 ± 4.9% and 69.66 ± 4.7% at concentrations of 200 µg/mL and 300 µg/mL of yacon extract respectively. FBS (10%) reduced TIMP-1 expression by 37.97 ± 4.1%. In contrast, yacon extract increased the expression of TIMP-1 by 23.67 ± 3.5% and 44.33 ± 3.9% at concentrations of 200 µg/mL and 300 µg/mL, respectively.
Samallanthus sonchifolius otherwise known as yacon, is widely cultivated worldwide and has been found to contain numerous carbohydrate and secondary metabolites. Several recent studies have demonstrated the medical benefits of yacon, which are related to its effects on various cellular signaling molecules [12,13,14]. However, none of these studies has yet established the effect of yacon on brain tumors. To address this we have attempted to investigate the components of yacon and its effects in preventing the progression of glioma. This study suggests that yacon contains components that can prevent glioma progression.
Glioma is associated with aggressive tumor cell motility that contributes to the rapid metastasis of the cancer cells in to normal tissues. This leads to a lack of a distinct boundary between normal tissues and the tumor, limiting the possibility for effective surgery [2]. Martin et. al. have suggested that antioxidants may control glioma cell proliferation [19]. Campos et al. have shown that yacon has a high antioxidant content [20]. Although cancer progression is associated with complex signaling pathways, normally the phenomenon of metastasis involves migration of cells [21]. We explored the inhibitory effect of yacon on the migration of C6 glioma cells using noncytotoxic concentrations of yacon. Our data from the scratch wound healing assay and the Boyden chamber assay showed that yacon inhibited abnormal cell motility in FBS-induced C6 glioma cells in a dose-dependent manner.
The invasion and migration of malignant cells play an important role in the progression of cancer from one stage to another [22]. Recent advances in cancer therapy suggest that controlling cell migration by targeting molecular function is a promising treatment strategy [23]. ERK1/2 contributes to cell migration and proliferation in a number of different types of cancer cell [6]. Recent extensive analysis of fetal bovine serum (FBS) has demonstrated that it contains complex components not present in newborn calf serum (NCS). These active components are believed to directly influence the proliferation and differentiation of cells [24], as well as activate signaling pathways, such as MAPK signaling pathway, involved in cell migration and invasion [25]. A number of studies have shown that specific growth factors directly influence cell mobility patterns found in a number of different cancers including lung cancer and glioma [26,27,28]. Antioxidant proteins, which are activated via tyrosine kinase receptor (RTK) pathways, are known to counter-regulate abnormal cell growth in tumor cells. For instance, N-acetylcysteine (NAC) has been shown to regulate the proliferation of glioma cells by regulating cell cycle arrest at the G0/G1 transition and by up-regulating expression of p21 [16]. In this study, we examined the effects of yacon on C6 glioma cells and tested whether it could suppress the phosphorylation of ERK1/2 a component of the MAPK pathway. Our results show that yacon significantly decreased the phosphorylation of ERK1/2 in FBS-stimulated C6 glioma cells. The major finding of our study is that the suppression of cell migration in FBS-stimulated C6 glioma cells by yacon is mediated by the ERK1/2 pathway. Therefore, these results suggest that yacon is a potential functional food candidate that can act as an antioxidant and is therefore potentially useful in preventing glioma progression.
Furthermore, we found that the expression of MMP9 was significantly inhibited by yacon in a concentration-dependent manner. MMP9 interacts with major intracellular signal transduction pathways causing the extracellular matrix (ECM) destruction and is a well-known regulator of the cancer cell migration pathway [10]. For these reasons, we investigated the concentration of yacon required to inhibit or reduce the expression of MMP9. We also tested whether yacon could induce TIMP-1 expression. Interestingly, the expression levels of TIMP-1 were also reduced by yacon in a concentration-dependent manner.
In conclusion, we suggest that yacon is a potential candidate for the treatment of malignant gliomas because of its ability to significantly reduce the motility of C6 glioma cells. This effect was found to occur through modulation of the phosphorylation of ERK1/2 and regulation of MMP9/TIMP-1 signaling. The major finding of our study is that yacon contributes to the suppression of cell migration of FBS-stimulated C6 glioma through inhibition of the ERK1/2 and MMP9/TIMP-1 signaling pathways. However, based on the present data, we cannot specify the exact amount of yacon intake required to prevent the migration of glioma in the brain. Because our data was obtained in vitro, further in vivo experiments are required to confirm these results.
Figures and Tables
 | Fig. 1The effect of yacon extract on the cell viability of C6 glioma cells.C6 glioma cells were seeded and incubated in serum free media for 24 h. Post-treatment cell viabilities were evaluated in the presence or absence of 10% FBS. The XTT assay was used to measure viabilities at an absorbance of 450 nm. The data is presented as mean ± SE (n = 3). Values with the same superscript letter are not significantly different based on Tukey's multiple range test (P < 0.05).
|
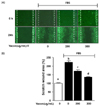 | Fig. 2The effect of yacon extract on FBS-stimulated C6 glioma cells.(A) The cells were seeded and incubated in serum free media for 24 h. The scratch was made by using a 200-µL pipette tip and the post-treatment effect of yacon extract at 200 µg/mL and 300 µg/mL on cell motility was determined. (B) A graph of the scratch wound data. Results are mean ± SE (n = 3). Values with the same superscript letter are not significantly different using Tukey's multiple range test (P < 0.05).
|
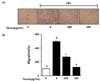 | Fig. 3The effect of yacon extract on FBS-induced migration of C6 glioma cells.C6 glioma cells were seeded and incubated in serum free media for 24 h. The cells were harvested with trypsin. C6 cells were loaded in the upper chamber and yacon extract (200 µg/mL and 300 µg/mL) was loaded in the lower chamber. The motility of the cells treated with yacon extract was determined relative to the control group (untreated FBS and yacon extract). The bars represent means ± SE (n = 3). Values with the same superscript letter are not significantly different, as determined using Tukey's multiple range test (P < 0.05).
|
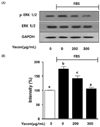 | Fig. 4The effect of yacon extract on FBS-stimulated phosphorylation of ERK1/2 in C6 glioma cell.(A) C6 glioma cells were seeded and then incubated in serum free media for 24 h. Cells were pre-treated with different concentrations of yacon (200 µg/mL and 400 µg/mL) for 1 h, and then stimulated with 10% FBS for 30 min. The expression levels of p-ERK 1/2 in cell lysates were examined by immunoblot analysis. (B) The graph shows the intensity of the bands relative to the untreated group. Results are presented as means ± SE, and are represent 3 independent experiments. Values with the same superscript letter are not significantly different, as determined by Tukey's multiple range test (P < 0.05).
|
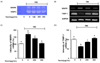 | Fig. 5The effect of yacon extract on MMPs and TIMP-1 activity in FBS-stimulated C6 glioma cells.C6 glioma cells were seeded and then incubated in serum-free media for 24 h. (A) Cells were pre-treated with different concentrations of yacon for 1 h, and then treated with 10% FBS in addition to yacon for 24 h. Zymogarphy analysis were performed as described in material and methods session. (B) Cells were pre-treated with different concentrations of yacon for 1 h, and then treated with 10% FBS in addition to yacon for 6 h. Then, the RNA extraction and RT-PCR analysis were performed as described in material and methods session. (C,D) The graph represents the intensity of the bands relative to the untreated group. Results are presented as mean ± SE, and are represent 3 independent experiments. Values with the same superscript letter are not significantly different, as determined by Tukey's multiple range test (P < 0.05).
|
References
1. Chinot OL, Macdonald DR, Abrey LE, Zahlmann G, Kerloëguen Y, Cloughesy TF. Response assessment criteria for glioblastoma: practical adaptation and implementation in clinical trials of antiangiogenic therapy. Curr Neurol Neurosci Rep. 2013; 13:347.

2. Hess KR, Broglio KR, Bondy ML. Adult glioma incidence trends in the United States, 1977-2000. Cancer. 2004; 101:2293–2299.

3. Marucci G. The effect of WHO reclassification of necrotic anaplastic oligoastrocytomas on incidence and survival in glioblastoma. J Neurooncol. 2011; 104:621–622.

4. Coniglio SJ, Segall JE. Review: molecular mechanism of microglia stimulated glioblastoma invasion. Matrix Biol. 2013; 32:372–380.

5. Roy LO, Poirier MB, Fortin D. Transforming growth factor-beta and its implication in the malignancy of gliomas. Target Oncol. Forthcomng 2014.

6. Strnisková M, Barancík M, Ravingerová T. Mitogen-activated protein kinases and their role in regulation of cellular processes. Gen Physiol Biophys. 2002; 21:231–255.
8. Park SL, Kim WJ, Moon SK. p21WAF1 mediates the IL-15-induced migration and invasion of human bladder cancer 5637 cells via the ERK1/2/NF-kappaB/MMP-9 pathway. Int Immunopharmacol. 2014; 22:59–65.

9. Khasigov PZ, Podobed OV, Gracheva TS, Salbiev KD, Grachev SV, Berezov TT. Role of matrix metalloproteinases and their inhibitors in tumor invasion and metastasis. Biochemistry (Mosc). 2003; 68:711–717.
10. Fang W, Li H, Kong L, Niu G, Gao Q, Zhou K, Zheng J, Wu B. Role of matrix metalloproteinases (MMPs) in tumor invasion and metastasis: serial studies on MMPs and TIMPs. Beijing Da Xue Xue Bao. 2003; 35:441–443.
11. Deryugina EI, Quigley JP. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev. 2006; 25:9–34.

12. Delgado GT, Tamashiro WM, Maróstica MR Junior, Pastore GM. Yacon (Smallanthus sonchifolius): a functional food. Plant Foods Hum Nutr. 2013; 68:222–228.

13. Genta S, Cabrera W, Habib N, Pons J, Carillo IM, Grau A, Sánchez S. Yacon syrup: beneficial effects on obesity and insulin resistance in humans. Clin Nutr. 2009; 28:182–187.

14. de Almeida Paula HA, Abranches MV, de Luces Fortes Ferreira CL. Yacon (Smallanthus sonchifolius): a food with multiple functions. Crit Rev Food Sci Nutr. 2015; 55:32–40.

15. Piette C, Deprez M, Roger T, Noël A, Foidart JM, Munaut C. The dexamethasone-induced inhibition of proliferation, migration, and invasion in glioma cell lines is antagonized by macrophage migration inhibitory factor (MIF) and can be enhanced by specific MIF inhibitors. J Biol Chem. 2009; 284:32483–32492.

16. Kobayashi T, Hattori S, Shinkai H. Matrix metalloproteinases-2 and -9 are secreted from human fibroblasts. Acta Derm Venereol. 2003; 83:105–107.

17. Delgado GT, Thomé R, Gabriel DL, Tamashiro WM, Pastore GM. Yacon (Smallanthus sonchifolius)-derived fructooligosaccharides improves the immune parameters in the mouse. Nutr Res. 2012; 32:884–892.

18. Lin F, Hasegawa M, Kodama O. Purification and identification of antimicrobial sesquiterpene lactones from yacon (Smallanthus sonchifolius) leaves. Biosci Biotechnol Biochem. 2003; 67:2154–2159.

19. Martín V, Herrera F, García-Santos G, Antolín I, Rodriguez-Blanco J, Rodriguez C. Signaling pathways involved in antioxidant control of glioma cell proliferation. Free Radic Biol Med. 2007; 42:1715–1722.

20. Campos D, Betalleluz-Pallardel I, Chirinos R, Aguilar-Galvez A, Noratto G, Pedreschi R. Prebiotic effects of yacon (Smallanthus sonchifolius Poepp. & Endl), a source of fructooligosaccharides and phenolic compounds with antioxidant activity. Food Chem. 2012; 135:1592–1599.

21. Friedl P, Wolf K. Tumour-cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer. 2003; 3:362–374.

22. Nagano M, Hoshino D, Koshikawa N, Akizawa T, Seiki M. Turnover of focal adhesions and cancer cell migration. Int J Cell Biol. 2012; 2012:310616.

23. Gialeli C, Theocharis AD, Karamanos NK. Roles of matrix metalloproteinases in cancer progression and their pharmacological targeting. FEBS J. 2011; 278:16–27.

24. Li C, Wang F, Liu L. Ultra-high performance liquid chromatography-mass spectrometry for analysis of newborn and fetal bovine serum components. Nan Fang Yi Ke Da Xue Xue Bao. 2014; 34:751–753.
25. Wang M, Maier P, Wenz F, Giordano FA, Herskind C. Mitogenic signalling in the absence of epidermal growth factor receptor activation in a human glioblastoma cell line. J Neurooncol. 2013; 115:323–331.

26. Ito Y, Correll K, Schiel JA, Finigan JH, Prekeris R, Mason RJ. Lung fibroblasts accelerate wound closure in human alveolar epithelial cells through hepatocyte growth factor/c-Met signaling. Am J Physiol Lung Cell Mol Physiol. 2014; 307:L94–L105.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download