Abstract
The liver is vulnerable to alcohol-related injury because it is the primary site of alcohol metabolism. Additionally, a number of potentially dangerous by-products are generated as alcohol is broken down in the liver. However, dietary supplements may prevent or relieve some of alcohol's deleterious effects. Therefore, this study was conducted to evaluate the prophylactic effect of aqueous extract of Sesamum indicum (SI) on ethanol induced toxicity in rats. Male Wistar albino rats were divided into control, ethanol, pre-treatment, simultaneous and post-treatment groups. In the prophylactic experiment, Sesamum indicum, (200 mg/kg body weight) was administered by oral gavage for 28 days; two hours before, simultaneously with or two hours after ethanol exposure. Toxicity was induced by administering 45% ethanol (4.8 g/kg bw) by oral gavage. Lipid peroxidation (TBARS) and reduced glutathione (GSH) levels and catalase (CAT), glutathione peroxidase (GPx), superoxide dismutase (SOD) and gluthathione-S-transferase (GST) activities were then determined in the liver, serum triglyceride (TG) levels, alanine aminotransferase (ALT) and aspartate aminotransferase (AST) activities were monitored and histological examination was carried out. The results revealed that ethanol administration led to significant elevation of TBARS level while depleting in the level of GSH as well as CAT, GPx, SOD and GST activities. Similarly, TG level and ALT and AST activities were elevated. The SI pre-treated group significantly inhibited TBARS, restored GSH level, enhanced CAT, GPx, SOD and GST activities and significantly decreased the elevated level of serum TG, ALT and AST activities. SI treatment (simultaneously with ethanol) exhibited similar effects to those of the SI pre-treated groups, while the SI post-treated group did not show the same protection as the Pre-treated group. S. indicum possesses antioxidant and hepatoprotective properties, that eliminate the deleterious effects of toxic metabolites of ethanol.
Metabolism of drugs and xenobiotics primarily occurs in the liver; therefore the liver is prone to xenobiotics-induced injury as a result of its pivotal role in xenobiotics metabolism. Alcohol is metabolized in the liver, which results in generation of a number of potentially dangerous by-products such as acetaldehyde and highly reactive free radicals that contribute to alcohol-induced liver damage [1,2]. Several studies have attempted to identify the molecular pathways, directly and indirectly affected by alcohol exposure in the liver. These pathways range from oxidative stress, metabolism-related effects, and inflammation to apoptosis. Induction of oxidative stress and activation of the inflammatory cascade have been identified as key elements in the pathophysiology of alcohol liver disease (ALD) [3-5].
The popularity of herbal remedies is increasing globally and at least one quarter of patients with liver diseases use ethnobotanicals [6]. Several plants and natural compounds isolated from plants have been shown to have hepatoprotective activities [7] and used to prevent the oxidative challenges against the liver during alcohol metabolism. It has been proposed that their efficacy is due to their free radical scavenging ability, which is believed to be associated with their bioactive and antioxidant constituents [8-10].
Sesamum indicum (SI), which is a plant with both medicinal and nutritive values, is popularly used as a herbal remedy against a wide range of ailments [11]. SI seed is not only a good source of edible nutrients, but is also widely considered to have medicinal value that includes antioxidant, anti-ageing, antimutagenic, antihypertensive, and anti-inflammatory activities, as well as the ability to inhibit cholesterol absorption from the intestine and synthesis in the liver. The seed is used as a duretic, emollient, and a tonic for the liver and kidneys [12,13].
SI is an annual shrub with white bell-shaped flowers that is grown for the production of seeds, which are rich in oil content. The plant is found in tropical, subtropical, and southern temperate areas of the world, particularly in India, China, South America and Africa [14]. This study was conducted to evaluate the prophylactic effects of aqueous extract of SI seeds on ethanol-induced toxicity in a rat model.
Thirty Male Wistar albino rats (weighing 180-200 g) were divided into control, ethanol, pre-treatment, simultaneous and post-treatment groups. The animals were allowed access to feed (obtained from Ladokun Feed Mill Nigeria Limited, Ibadan, Nigeria) and water ad libitum for fourteen days to allow their acclimatization prior to commencement of the experiment. A pilot study (unpublished data) showed that the aqueous extract was more potent than methanolic and hexane extracts at the tested concentrations (200 mg/kg bw). In the prophylactic experiment, SI (200 mg/kg bw) extract was administered by oral gavage for 28 days, two hours before, simultaneously with or two hours after ethanol exposure. Toxicity was induced by administering 45% ethanol (4.8 g/kg bw) by oral gavage. Animal experiments followed protocols established by the National Institute of Health (NIH) (NIH publication 85-23, 1985) for the Care and Use of Laboratory Animals and all procedures involving rats were conducted according to the ethical guidelines approved by the Animal Ethical Committee of Afe Babalola University (ABUAD-SCI03/13/09/002).
SI seeds were purchased from Ojoo market in Ibadan, Nigeria and then identified and authenticated by the Botany Department, University of Ibadan. One kilogram of the air-dried seeds was subsequently pulverized into uniform powder using an electric blender (25-28℃). Pulverized seed (1 kg) was then defatted by mixing with n-hexane (2000 ml) using a magnetic stirrer at room temperature for 6 hours. The resulting slurry was filtered and the residue was air dried for 24 hour. Next the dried defatted residue (600 g) was extracted with 1500 ml of distilled water by maceration for 72 hours. The aqueous extract subsequently was filtered and the filtrate was concentrated using water bath (80℃) to yield a brown extract. This was carefully scraped into a clean sample bottle and stored in a refrigerator until further use. Prior to use, detailed phytochemical screening of SI was carried out according to the method described by Harborne [15].
Randox alanine aminotransferase (ALT), aspartate aminotransferase (AST), and triglyceride (TG) assay kits were purchased from ABJ Chemicals, Lagos (Nigeria). Adrenaline, thiobarbituric acid (TBA), Ellman's reagent (DTNB), glutathione and bovine serum albumin (BSA) were purchased from Sigma Chemical (St. Louis, MO, USA). All other chemicals were of the highest purity commercially available.
Lipid peroxidation (LPO) was assayed by measuring thiobarbituric acid reactive substances (TBARS) as described by Varshney and Kale [16]. Catalase (CAT) activity was determined by measuring the rate of decomposition of hydrogen peroxide at 570 nm as described by Sinha [17]. SOD activity was determined as described by Misra and Fridovich [18].
Reduced glutathione (GSH) level was estimated using the method described by Beutler et al., [19] at 412 nm. GPx was determined by the method described by Hafeman et al. [20] based on the degradation of H2O2 in the presence of GSH. Glutathione-S-transferase (GST) activity was determined according to Habig et al. [21]. Serum ALT and AST activites and TG levels were quantified spectrophotometrically using a Randox commercial assay kit.
Following daily exposure for 28 days, the animals were sacrificed 24 hours after the last dose by cervical dislocation. Blood samples were then collected by retro-orbital puncture and allowed to coagulate at room temperature for half an hour, after which the serum was obtained by blood centrifugation at 3,000 rpm for 10 minutes and kept at 20℃ until analyses were done. Liver samples were quickly excised and washed in ice-cold 1.15% KCl solution, dried using filter paper and weighed. samples were then homogenized in 4 volumes of 56 mM Tris-HCl buffer (pH 7.4) containing 1.15% KCl, after which they were centrifuged at 10,000 g for 15 minutes.
The supernatant was collected and stored until needed for assays. Small pieces of liver sections were fixed in 10% formal saline, after which they were cut and stained with haematoxylin and eosin. The stained tissue sections were they observed under a light microscope (× 400 objective) for histological assessment.
The results of the phytochemical screening revealed the presence of the following metabolites in appreciable amount: flavonoids, saponins, tannins and phenols (Table 1).
The aqueous extract of SI did not show any sign or symptoms of toxicity and no mortality was recorded during the study. As shown in Table 2, TG, ALT and AST activities were found to increase in serum of the ethanol treated group relative to the control. Pre-treatment with SI two hours before ethanol exposure and simultaneous treatment with SI during ethanol exposure resulted in significant protection of the liver, as indicated by reductions in the elevated levels of TG, ALT and AST; however, there was no evidence of amelioration in the SI post-treated group.
SI pre-treatment prior to ethanol exposure caused a significant increase in GPx activities as well as a noticeable increase in GSH level while a significant reduction was observed in the level of TBARS, which is an indication of inhibition of lipid peroxidation. A similar result to that of SI pre-treated groups was observed in response to SI simultaneous treatment with ethanol, while the SI post-treated group did not show any significant protection (Table 3).
Table 4 presents the effects of SI on hepatic GST, SOD and CAT activities. Administration of SI before ethanol exposure and simultaneously with ethanol; enhanced the activities of these antioxidants, while there was no significant increase in the post-treated group relative to the control.
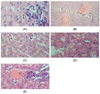
The histopathological alterations of liver tissue during ethanol-induced toxicity are shown in Fig. 1. The control liver showed normal liver histology, with no abnormalities. Multiple foci of congestion as well as massive and severe sinusoid infiltration by inflammatory cells were observed in the ethanol group. The SI pre-treated group showed very mild periportal cellular infiltration. Very mild diffuse hydropic degeneration of hepatocytes was observed in the SI simultaneous group, while the post-treated group showed mild accumulation of fats and infiltration of inflammatory cells.
Consumption of alcohol affects the liver and other organs and could contribute to the development of alcohol liver disease [22,23]. In the current study, the prophylactic effect of aqueous extract of SI seeds was investigated in ethanol-exposed rats, which is a widely used animal model. The results from a pilot study (unpublished data) showed that the aqueous extract was more potent than methanolic and hexane extracts at the tested concentrations (200 mg/kg bw). The presence of flavonoids, saponins, tannins and phenols in the aqueous extract used in the present study, may explain the effectiveness of the use of the aqueous extracts of SI in traditional medicine for the management of diseases.
We noticed that ethanol administration led to a significant increase in the level of serum triglyceride, which was attenuated by SI in the pre-treatment and simultaneous groups while there was no evidence of attenuation in the post-treatment group. Accumulation of fat is the earliest and most common response to heavy alcohol intake [1,24]. ALD is usually characterized by enlargement of the liver [25], increased serum and hepatic TG levels, and the presence of a high number of fat droplets in liver sections [26].
Well known biomarkers of liver injury (ALT and AST) and histological changes were examined to evaluate the prophylactic effects of SI. Consistent with previous studies, our study confirmed that acute ethanol exposure damaged the liver, as shown by elevation of the serum aminotransferase activities and morphological changes observed in the liver sections [27-29]. Interestingly, these adverse effects were significantly attenuated by SI in the pre-treatment and simultaneous groups, which indicated a prominent hepatoprotective effect of SI against ethanol toxicity, similar to that reported on Achyranthus Aspera L [30].
The observed elevation in the hepatic levels of TBARS is an indication of induction of lipid peroxidation [31,32]. This may be a result of the overwhelming effect of free radicals generated during ethanol metabolism [33]. The reduction in hepatic levels of TBARS observed in the pre-treatment and simultaneous groups could be attributed to the free radical scavenging properties and antioxidant activities of the extract that decreased the overload on the endogenous body antioxidants [34]. We also noticed that ethanol exposure led to impairment of the detoxication systems as observed by depletion of the level of GSH and the activities of other antioxidant enzymes (GPx, GST, SOD and CAT) [35-37]. GSH is an important constituent of cellular protective mechanisms in effecting detoxification of reactive metabolites from cells. The observed decrease in GSH level and other antioxidant enzymes activities might have been due to increased scavenging of reactive substances that were produced as a result of ethanol metabolism [38-39].
It has been well documented that enhancement of lipid peroxidation is a consequence of depletion of GSH to a certain critical level [40,41]. Administration of the extract before ethanol exposure and simultaneously with ethanol in the present study restored GSH level; and enhanced GPx, GST, SOD and CAT activities, which may be a reflection of their increased synthesis in the liver [42-43]. The possible mechanism by which SI brought about the observed changes in the present study may be synergy between different phytochemicals constituents present.
In conclusion, the results of the present study indicate that S. indicum possess antioxidant and hepatoprotective properties, eliminating the deleterious effects of toxic metabolites from ethanol when administered orally two hours before ethanol exposure or simultaneously with ethanol exposure.
Figures and Tables
Fig. 1
Histological analysis of liver sections. Liver tissues were stained with H&E (×400). (A) Control: showing normal liver histology, no abnormalities was seen. (B) Ethanol group: showing multiple foci of congestion, massive and severe sinusoid infiltration by inflammatory cells. (C) Pre-treatment group: showing very mild periportal cellular infiltration. (D) Simultaneous treatment group: showing very mild diffuse hydropic degeneration of hepatocytes. (E) Post-treatment group: showing mild accumulation of fats and infiltration of inflammatory cells.
References
1. Maher JJ. Exploring alcohol's effects on liver function. Alcohol Health Res World. 1997; 21:5–12.
2. Halliwell B, Gutteridge JM. The antioxidants of human extracellular fluids. Arch Biochem Biophys. 1990; 280:1–8.

3. Thurman RG, Bradford BU, Iimuro Y, Frankenberg MV, Knecht KT, Connor HD, Adachi Y, Wall C, Arteel GE, Raleigh JA, Forman DT, Mason RP. Mechanisms of alcohol-induced hepatotoxicity: studies in rats. Front Biosci. 1999; 4:e42–e46.
4. Gupta S, Pandey R, Katyal R, Aggarwal HK, Aggarwal RP, Aggarwal SK. Lipid peroxide levels and antioxidant status in alcoholic liver disease. Indian J Clin Biochem. 2005; 20:67–71.

5. Wu D, Cederbaum AI. Alcohol, oxidative stress, and free radical damage. Alcohol Res Health. 2003; 27:277–284.
6. Saleem TS, Chetty CM, Ramkanth S, Rajan VS, Kumar KM, Gauthaman K. Hepatoprotective herbs-a review. Int J Res Pharm Sci. 2010; 1:1–5.
7. Adewusi EA, Afolayan AJ. A review of natural products with hepatoprotective activity. J Med Plant Res. 2010; 4:1318–1334.
8. Kono H, Arteel GE, Rusyn I, Sies H, Thurman RG. Ebselen prevents early alcohol-induced liver injury in rats. Free Radic Biol Med. 2001; 30:403–411.

9. Wheeler MD, Nakagami M, Bradford BU, Uesugi T, Mason RP, Connor HD, Dikalova A, Kadiiska M, Thurman RG. Overexpression of manganese superoxide dismutase prevents alcohol-induced liver injury in the rat. J Biol Chem. 2001; 276:36664–36672.

10. Rosengren RJ. Catechins and the treatment of breast cancer: possible utility and mechanistic targets. IDrugs. 2003; 6:1073–1078.
11. Hajimahmoodi M, Oveisi MR, Sadeghi N, Jannat B, Bahaeddin Z, Mansoori S. Gamma tocopherol content of Iranian sesame seeds. Iran J Pharm Res. 2008; 7:135–139.
12. Uthandi A, Ramasamy K. Hepatoprotective activity of sesame meal on high fat fed wistar rats. Int J Pharm Sci Res. 2011; 2:205–211.

13. Utsunomiya T, Shimada M, Rikimaru T, Hasegawa H, Yamashita Y, Hamatsu T, Yamasaki M, Kaku S, Yamada K, Sugimachi K. Antioxidant and anti-inflammatory effects of a diet supplemented with sesamin on hepatic ischemia-reperfusion injury in rats. Hepatogastroenterology. 2003; 50:1609–1613.
14. Chakraborthy GS, Sharma G, Kaushik KN. Sesamum indicum: a review. J Herb Med Toxicol. 2008; 2:15–19.
15. Harborne JB. Organic acids, lipids and related compounds. Phytochemical Methods: A Guide to Modern Techniques of Plant Analysis. London: Chapman and Hall Ltd.;1973. p. 149–188.
16. Varshney R, Kale RK. Effects of calmodulin antagonists on radiation-induced lipid peroxidation in microsomes. Int J Radiat Biol. 1990; 58:733–743.

18. Misra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972; 247:3170–3175.

19. Beutler E, Duron O, Kelly BM. Improved method for the determination of blood glutathione. J Lab Clin Med. 1963; 61:882–888.
20. Hafeman DG, Sunde RA, Hoekstra WG. Effect of dietary selenium on erythrocyte and liver glutathione peroxidase in the rat. J Nutr. 1974; 104:580–587.

21. Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974; 249:7130–7139.
22. Tome S, Lucey MR. Review article: current management of alcoholic liver disease. Aliment Pharmacol Ther. 2004; 19:707–714.
23. Zeng MD, Li YM, Chen CW, Lu LG, Fan JG, Wang BY, Mao YM. Chinese National Consensus Workshop on Nonalcoholic Fatty Liver Disease. Guidelines for the diagnosis and treatment of alcoholic liver disease. J Dig Dis. 2008; 9:113–116.

24. Adiels M, Olofsson SO, Taskinen MR, Borén J. Overproduction of very low-density lipoproteins is the hallmark of the dyslipidemia in the metabolic syndrome. Arterioscler Thromb Vasc Biol. 2008; 28:1225–1236.

25. Song Z, Zhou Z, Deaciuc I, Chen T, McClain CJ. Inhibition of adiponectin production by homocysteine: a potential mechanism for alcoholic liver disease. Hepatology. 2008; 47:867–879.

26. Zeng T, Guo FF, Zhang CL, Zhao S, Dou DD, Gao XC, Xie KQ. The anti-fatty liver effects of garlic oil on acute ethanol-exposed mice. Chem Biol Interact. 2008; 176:234–242.

27. Adewusi EA, Afolayan AJ. Effect of Pelargonium reniforme roots on alcohol-induced liver damage and oxidative stress. Pharm Biol. 2010; 48:980–987.

28. Nwozo SO, Oyinloye BE. Hepatoprotective effect of aqueous extract of Aframomum melegueta on ethanol-induced toxicity in rats. Acta Biochim Pol. 2011; 58:355–358.

29. Tabassum F, Khurshid R, Karim S, Akhtar MS. Metabolic effects of alcoholism and its relationship with alcoholic liver disease. J Ayub Med Coll Abbottabad. 2001; 13:19–21.
30. Sudha A, Srinivasan P, Manikandaselvi S, Thinagarbabu R. Protective effect and antioxidant role of Achyranthus Aspera L. against ethanol-induced oxidative stress in rats. Int J Pharm Pharm Sci. 2012; 4:Suppl 3. 280–284.
31. Crews FT, Nixon K. Mechanisms of neurodegeneration and regeneration in alcoholism. Alcohol Alcohol. 2009; 44:115–127.

32. De Freitas V, Da Silva Porto P, Assunção M, Cadete-Leite A, Andrade JP, Paula-Barbosa MM. Flavonoids from grape seeds prevent increased alcohol-induced neuronal lipofuscin formation. Alcohol Alcohol. 2004; 39:303–311.

34. Reddy VD, Padmavathi P, Paramahamsa M, Varadacharyulu NC. Amelioration of alcohol-induced oxidative stress by Emblica officinalis (amla) in rats. Indian J Biochem Biophys. 2010; 47:20–25.
35. Curtis SJ, Moritz M, Snodgrass PJ. Serum enzymes derived from liver cell fractions. I. The response to carbon tetrachloride intoxication in rats. Gastroenterology. 1972; 62:84–92.
36. Geesin JC, Hendricks LJ, Falkenstein PA, Gordon JS, Berg RA. Regulation of collagen synthesis by ascorbic acid: characterization of the role of ascorbate-stimulated lipid peroxidation. Arch Biochem Biophys. 1991; 290:127–132.

37. Rukkumani R, Aruna K, Varma PS, Menon VP. Influence of ferulic acid on circulatory prooxidant-antioxidant status during alcohol and PUFA induced toxicity. J Physiol Pharmacol. 2004; 55:551–561.
39. Lieber CS. Role of oxidative stress and antioxidant therapy in alcoholic and nonalcoholic liver diseases. Adv Pharmacol. 1997; 38:601–628.

40. Nordmann R. Alcohol and antioxidant systems. Alcohol Alcohol. 1994; 29:513–522.
41. Altomare E, Grattagliano I, Vendemiale G, Palmieri V, Palasciano G. Acute ethanol administration induces oxidative changes in rat pancreatic tissue. Gut. 1996; 38:742–746.

42. Esmaeili MA, Sonboli A, Kanani MR, Sadeghi H. Salvia sahendica prevents tissue damages induced by alcohol in oxidative stress conditions: effect on liver and kidney oxidative parameters. J Med Plant Res. 2009; 3:276–283.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download