Abstract
BACKGROUND/OBJECTIVES
D. candidum is a traditional Chinese food or medicine widely used in Asia. There has been little research into the anticancer effects of D. candidum, particularly the effects in colon cancer cells. The aim of this study was to investigate the anticancer effects of D. candidum in vitro and in vivo.
MATERIALS/METHODS
The in vitro anti-cancer effects on HCT-116 colon cancer cells and in vivo anti-metastatic effects of DCME (Dendrobium canidum methanolic extract) were examined using the experimental methods of MTT assay, DAPI staining, flow cytometry analysis, RT-PCR, and Western blot analysis.
RESULTS
At a concentration of 1.0 mg/mL, DCME inhibited the growth of HCT-116 cells by 84%, which was higher than at concentrations of 0.5 and 0.25 mg/mL. Chromatin condensation and formation of apoptotic bodies were observed in cancer cells cultured with DCME as well. In addition, DCME induced significant apoptosis in cancer cells by upregulation of Bax, caspase 9, and caspase 3, and downregulation of Bcl-2. Expression of genes commonly associated with inflammation, NF-κB, iNOS, and COX-2, was significantly downregulated by DCME. DCME also exerted an anti-metastasis effect on cancer cells as demonstrated by decreased expression of MMP genes and increased expression of TIMPs, which was confirmed by the inhibition of induced tumor metastasis in colon 26-M3.1 cells in BALB/c mice.
Dendrobium, microspermae, is a perennial epiphytic herb in the family Orchidaceae [1]. D. candidum is unique in its medicinal value. Its stem can be used as medicine, which can promote humoral secretion, prevent development of cataracts, relieve guttural agnail and fatigue, reduce peripheral vascular blockage, and improve immune function [2]. D. candidum can also be used in health care products, which are effective for toning the stomach, promoting hydration, nourishing yin, and reducing body heat. As a kind of traditional Chinese health product and Chinese medical herb, it has high value in use [3].
Apoptosis is an important cellular defense against cancer [4] and caspases form the central components of an apoptotic response. Nuclear factor-κB (NF-κB) is involved in the inhibition of apoptosis, stimulation of cell proliferation, inflammation, immune response, and tumorigenesis. Expression of inducible nitric oxide synthase (iNOS) and cyclooxygenase 2 (COX-2), two genes regulated by NF-kB, is induced by inflammation and they are frequently over-expressed in cancer cells [5]. Metastasis, the leading cause of death among cancer patients, involves the spread of cancer from a primary site and formation of new tumors in distant organs. Matrix metalloproteases (MMPs) play important roles in many physiological or pathological processes involved in metastasis. MMP activity is inhibited by specific endogenous tissue inhibitors of metalloproteinases (TIMPs) [6].
Previously, Bao et al. found that D. candidum produces strong in vitro anti-cancer effects on HeLaS3 human cervix carcinoma cells and HepG2 liver cancer cells [7]. In the current study, we further examined the anti-cancer and anti-metastatic effects of D. candidum. HCT-116 human colon cancer cells were treated with DCME (Dendrobium canidum methanolic extract) and the molecular mechanisms underlying the anti-cancer effects of DCME were studied. We evaluated DCME using different concentrations and also assessed its anti-metastatic effects in mice with tumors propagated by 26-M3.1 colon carcinoma cells.
D. candidum was purchased in Yunnan, China. It was stored at -80℃ and freeze-dried to produce a powder. The powdered sample was extracted with 20-fold volume of methanol twice overnight. The methanol extract was evaporated using a rotary evaporator (Eywla, N-1100, Tokyo, Japan), concentrated, and then dissolved in dimethyl sulfoxide (DMSO; Amresco, Soion, OH, USA) to adjust the stock concentration (20%, w/v) and named as DCME for future reference.
HCT-116 human colon carcinoma cells obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA) were used for experiments. The cells were cultured in RPMI-1640 medium (Gibco Co., Birmingham, MI, USA supplemented with 10% fetal bovine serum (FBS; Gibco Co.) and 1% penicillin-streptomycin (Gibco Co.) at 37℃ in a humidified atmosphere containing 5% CO2 (Forma, model 311 S/N29035; Waltham, MA, USA). The medium was changed two or three times each week.
26-M3.1 colon carcinoma cells were obtained from Prof. Yoon, Department of Food and Nutrition, Yuhan University, Bucheon, South Korea. These highly metastatic cells were maintained as monolayers in Eagle's minimal essential medium (EMEM) (Welgene Inc., Daegu, South Korea) supplemented with 7.5% FBS, a vitamin solution, sodium pyruvate, non-essential amino acids, and L-glutamine (Gibco Co.). The cultures were maintained in a humidified atmosphere of 5% CO2 at 37℃. Experimental lung metastasis was induced by injection of colon 26-M3.1 cells into the lateral tail vein of 6-wk-old female Balb/c mice (Hyochang Science, Daegu, South Korea) [8]. DCME solutions (50, 100, and 200 mg/kg) were administered by subcutaneous injection into the mice, and the animals then received intravenous inoculation with 26-M3.1 cells (2.5 × 104/mouse) after 2 d. After 2 wk the mice were sacrificed and their lungs were fixed in Bouin's solution (saturated picric acid: formalin: acetic acid; 15:5:1, v/v/v). The rate of metastasis was assessed by counting the lung tumor colonies using a digital camera (Canon D550, Tokyo, Japan). The protocol for these experiments was approved by the Animal Ethics Committee of Chongqing Medical University.
Anti-cancer effects of DCME were assessed by MTT assay [9]. HCT-116 human colon cancer cells were seeded in a 96-well plate at a density of 2 × 104 cells/mL in a volume 180 µL per well. DCME at final concentrations of 0.25, 0.5, and 1.0 mg/mL, 20 µL was added, and the cells were then incubated at 37℃ in 5% CO2 for 48 h. Next, MTT solution (200 µL, 5 mg/mL; Amresco, Solon, OH, USA) was added and the cells were cultured for another 4 h under the same conditions. After removing the supernatant, 150 µL of DMSO was added per well and mixed for 30 min. Finally, the absorbance of each well was measured using an ELISA reader (model 680; Bio-Rad, Hercules, CA, USA) at 540 nm.
Untreated control cells and cells treated with the DCME were harvested, washed with PBS, and fixed with 3.7% paraformaldehyde (Sigma, St. Louis, MO, USA) in PBS for 10 min at room temperature. The fixed cells were washed with PBS and stained with a 1 mg/mL DAPI (Sigma) solution for 10 min at room temperature. The cells were washed two more times with PBS and examined using a fluorescence microscope (BX50; Olympus, Tokyo, Japan).
After treatment with DCME the cells were trypsinized, collected, washed with cold PBS, and resuspended in 2 ml PBS. DNA contents of the cells were measured using a DNA staining kit (CycleTEST™ PLUS kit; Becton Dickinson, Franklin Lakes, NJ, USA). Nuclear fractions stained with propidium iodide were obtained by following the manufacturer's protocol. Fluorescence intensity was determined using a FACScan flow cytometer (EPICS XL-MCL, Beckman Coulter KK, Brea, CA, USA) and analyzed using CellQuest software (Becton Dickinson, Franklin Lakes, NJ, USA).
HCT-116 cells were inoculated in a 10 cm culture dish. After managing them according to the method of the MTT experiment for 24 h, total RNA in cells was extracted using Trizol according to the instructions. The total RNA concentration from each sample group was adjusted to the same level after testing its purity with ultraviolet radiation. The same amount of RNA (2 µg) was taken from the samples, followed by addition of 1 µL oligodT18, RNase, dNTP and 5 × buffer 10 µL enzyme MLV, respectively. In 20 µL body fluid, cDNA was synthetized at 37℃ for 120 min and 99℃ for 4 min and 4℃ for 3 min, respectively. The target genes were then reverse transcribed and amplified (Table 1). The reaction conditions were initial denaturation for 5 min at 95℃, annealing for 50s at 58℃, extension for 90 s at 72℃, then repeating it 40 times, and extension for 10 min at 95℃. In the end, 2% agarose gel electrophoresis was performed to determine expression of the final products [10].
After DCME treatment, protein lysates were added to the HCT-116 cancer cells after rinsing with pre-cooled PBS three times, lysed at 4℃ and centrifuged (10000 r/min) for 15 min. Supernatant proteins were then extracted and mixed with SDS-PAGE loading buffer. Primary antibodies were then applied to them after SDS-PAGE gel electrophoresis, followed by transfer to a membrane, and the proteins were incubated overnight at 4℃. Then the proteins were incubated with horseradish peroxidase-conjugated secondary antibodies at room temperature. In the end, immunoreactive proteins were tested using a chemiluminescent enhanced chemiluminescence assay kit and observed using a LAS3000 luminescent image analyzer with β-actin as an internal reference [11].
Data are presented as the mean ± SD. Differences between the mean values for individual groups were assessed using one-way ANOVA and Duncan's multiple range tests. Differences were considered significant when P < 0.05. SAS version 9.1 (SAS Institute Inc., Cary, NC, USA) was used for statistical analysis.
The in vitro anticancer effects of the D. candidum methanolic extract (DCME) were evaluated using the MTT assay, DAPI and flow cytometric analysis, gene expression analysis by RT-PCR, and Western blotting in HCT-116 cancer cells. The results showed that DCME had a strong in vitro as well as in-vivo anticancer activity and increased with increasing concentration. The component analysis explained the mechanism of DCME action.
Prophylactic inhibition of tumor metastasis by DCME was evaluated using an experimental mouse metastasis model (Table 2). All DCME-treated mice had significantly fewer lung metastatic colonies than control mice (number of metastatic tumors, 57 ± 6, n = 10; P < 0.05). DCME was the most effective for inhibition of lung metastasis at a concentration of 200 mg/kg. At this concentration tumor formation and lung metastasis were inhibited to a greater degree compared with the 100 mg/kg and 50 mg/kg doses.
Anti-cancer effects of DCME on HCT-116 cells were evaluated using a MTT assay. The survival rates of HCT-116 human colon cancer cells treated with different concentrations of DCME are shown in Table 3. HCT-116 cells were treated with different concentrations of D. candidum (0.25, 0.5, and 1.0 mg/mL) with cancer cell survival rates of 69%, 41%, and 16%, respectively (P < 0.05). These results demonstrated that DCME had significant anti-proliferative effects on HCT-116 cells. In addition, 1.0 mg/mL of DCME showed the strongest anticancer effects in vitro.
Induction of apoptosis was monitored to determine a possible mechanism underlying the inhibitory activity of DCME on HCT-116 cancer cells. The extent of chromatin condensation was analyzed by fluorescence microscopy of cells stained with the DNA-binding fluorescent dye DAPI and flow cytometric analysis. While untreated HCT-116 cells presented nuclei with homogeneous chromatin distribution, treatment with DCME induced chromatin condensation and nuclear fragmentation, suggesting the presence of apoptotic cells (Fig. 1A). Chromatin condensation and formation of apoptotic bodies, the two hallmarks of apoptosis, were observed in cells treated with 1.0 mg/mL DCME. In contrast, the level of chromatin condensation was low in cells treated with 0.25 or 0.5 mg/mL DCME. Flow cytometric analysis showed that treatment with 1.0 mg/mL D. candidum promoted apoptosis of HCT-116 cells more strongly when compared to lower concentrations of 0.25 and 0.5 mg/mL of DCME (P <0.05). This conclusion was based on the significant accumulation of cells with sub-G1 DNA content (Fig. 1B).
To elucidate the mechanisms underlying the inhibition of cancer cell growth by DCME, expression of Bax, Bcl-2, and caspase-3 and -9 in HCT-116 human colon cancer cells was measured by RT-PCR and western blot analyses after incubation with different concentrations of DCME for 48 h. As shown in Fig. 2, expression of pro-apoptotic Bax and anti-apoptotic Bcl-2 showed significant changes in the presence of 1.0 mg/mL DCME. These results suggest that DCME induced apoptosis in HCT-116 cells via a Bax- and Bcl-2-dependent pathway. The mRNA and protein expression levels of caspase-3 and -9 were very low in untreated control HCT-116 cells, but increased significantly after the cells were treated with 1.0 mg/mL of D. candidum. With the D. candidum treatment, mRNA and protein expression of caspase-3 and -9 was gradually elevated with increased concentrations (Fig. 2). More specifically, induction of apoptosis by DCME was related to upregulation of Bax, caspase-3, and caspase-9, and downregulation of Bcl-2 in terms of mRNA and protein expression. The effects of 1.0 mg/mL DCME were greater compared to those of the 0.25 and 0.5 mg/mL D. candidum solutions.
We attempted to determine whether the anti-cancer actions of DCME were associated with NF-κB, IκB-α, iNOS, and COX-2 gene expression. As shown in Fig. 3, mRNA and protein expression of NF-κB and IκB-α was reduced in HCT-116 cells treated with a 1.0 mg/mL D. candidum solution. D. candidum significantly modulated the expression of genes associated with inflammation. mRNA and protein expression of NF-κB were decreased while IκB-α mRNA levels were increased. In addition, mRNA and protein expression of COX-2 and iNOS showed a gradual decrease in the presence of DCME in a concentration dependent manner (Fig. 3). Our findings indicate that DCME may be helpful in prevention of cancer in early stages by increasing anti-inflammatory activities. Overall, the results of this experiment showed that 1.0 mg/mL DCME had a stronger anti-inflammatory effect on colon cancer cells than 0.25 and 0.5 mg/mL concentrations.
RT-PCR and western blot analyses were performed to determine whether the anti-metastatic effect of D. candidum was due to gene regulation of metastatic mediators, specifically MMPs (MMP-2 and MMP-9) and TIMPs (TIMP-1 and TIMP-2), in HCT-116 cells. As shown in Fig. 4, 1.0 mg/mL DCME induced a significant decrease in mRNA and protein expression of MMP-2 and MMP-9, and increased the expression of TIMP-1 and TIMP-2. These changes in TIMP and MMP expression resulting from DCME treatment could effectively lead to metastatic inhibition in vitro. Our results also showed that 1.0 mg/mL DCME has the strongest anti-metastatic activity.
Although D. candidum has been used as a medicine or functional food, little scientific data on its effects are available. D. candidum contains high concentrations of benzenes and their derivatives, phenolic, lignans, lactone, flavonoids, and 18 types of novel pigments were also found [12]. D. candidum has recently been reported to have various therapeutic effects on numerous pathologic conditions, including inflammation, immunity, hpyerglycemic, and cancer [13]. A previous study using the MTT assay in HeLaS3 human cervix carcinoma cells and HepG2 liver cancer cells reported on the in vitro anticancer effects of D. candidum [7]. In this study, it was also proven that DCME has inhibitory effects by MTT assay and in vitro anticancer effects using DAPI assay, flow cytometry, RT-PCR, and Western blot.
Apoptosis is programmed cell death, a process implemented by cells themselves for their own physiological and pathological factors [14]. In a healthy cell, the anti-apoptotic protein Bcl-2 is expressed on the outer mitochondrial membrane surface [15]. After treatment of HCT-116 cells with DCME, we observed that the number of apoptotic cancer cells increased after treatment with a high concentration of DCME, as seen with the DAPI and flow cytometry assays. Because the Bax and Bcl-2 genes are mainly expressed during apoptosis, we determined that these genes regulated the apoptotic activity. Apoptosis results from activation of caspase family members that act as aspartate-specific proteases [16]. Caspases are a type of protease hydrolysate which usually exists in the form of pro-caspase. However, among these pro-caspases, caspase-3 and caspase-9 are the main protease hydrolysates involved in the process of apoptosis. Caspase-9, an upstream caspase, acts as an apoptosis-trigger caspase, responsible for activation of downstream caspases. Caspase-3 is a downstream caspase that induces apoptosis via hydrolyzing apoptosis-effect molecules. Hydrolysis activates caspase-3 and these active caspases induce apotosis [17]. In this study, the gene and protein expressions of Bax, caspase-3, and caspase-9 increased, while expression of Bcl-2 decreased after treatment with DCME. Based on previous studies on gene expression, DCME may be assumed to be a strong inducer of apoptosis in cancer cells.
In addition, the anti-cancer mechanisms underlying the effect of DCME on human cancer cells involve the induction of apoptosis by increasing the number of apoptotic bodies, regulating the mRNA and protein expression of Bax and Bcl-2, and promoting anti-inflammatory effects by downregulating iNOS and COX-2 gene expression. COX-2 has been suggested to play an important role in colon carcinogenesis, and NOS, along with iNOS, may be a good target for chemoprevention of colon cancer [18]. NF-κB, one of the most ubiquitous transcription factors, regulates the expression of genes required for cellular proliferation, inflammatory responses, and cell adhesion [19]. NF-κB is present in the cytosol where it is bound to the inhibitory protein, IκB. Following its induction by a variety of agents, NF-κB is released from IκB and translocates to the nucleus where it binds to κB binding sites in the promoter regions of target genes [20]. These mechanisms could be involved in the anti-cancer effects of DCME in colon cancer cells. Based on the results of the MTT assay and the expression patterns of pro-apoptotic genes observed in the current study, we concluded that cancer cells treated with DCME underwent apoptosis. Similar to our findings, the anti-cancer effects of DCME in HeLaS3 human cervix carcinoma cells and HepG2 liver cancer cells were evaluated in a previous study by MTT assay [10].
Metastasis is defined as the spread of cancer cells from one organ or area to another adjacent organ or location [21]. It is thought that malignant tumor cells have the capacity to metastasize. Cancer occurs after cells in a tissue are genetically damaged in a progressive manner, resulting in cancer stem cells possessing a malignant phenotype. Once the tumor cells have come to rest in another site, they penetrate the vessel walls, continue to multiply, and eventually form another tumor. Colon 26-M3.1 carcinoma cells have been used in evaluation of anti-metastasis effects in vivo [22]. Based on the in vitro test results like the previous studies, the colon 26-M3.1 carcinoma cell anti-metastasis mice test was used to examine DCME. These results further proved the activities of D. candidum, and the anticancer effect and concentration were directly related.
MMPs, a family of zinc-dependent endopeptidases, play a very important role in tumorigenesis and metastasis. MMPs can cleave virtually all extracellular matrix (ECM) substrates. Degradation of the ECM is a key event in tumor progression, invasion, and metastasis. Among the MMP family members, MMP-2 and MMP-9 are important molecules for cancer invasion, and are highly expressed in breast and colon cancer cells [23]. In fact, inhibition of MMP activity is useful for controlling tumorigenesis and metastasis. TIMPs are naturally occurring inhibitors of MMPs which prevent catalytic activity by binding to activated MMPs, thereby blocking ECM breakdown. Disturbances in the ratio between MMPs and TIMPs have been observed during tumorigenesis. Maintaining the balance between MMPs and TIMPs or increasing TIMP activity is an effective way to control tumor metastasis [24]. Experimental evidence demonstrating the role of MMPs in metastasis has been obtained by in vitro invasion assays and in vivo xenograft metastasis experiments. MMP-2 and -9 are key factors in cancer cell invasion and metastasis both in vivo and in vitro. Spontaneous and experimental metastasis to the liver is decreased in mice overexpressing TIMP1, and increased in mice expressing antisense TIMP-1 mRNA. Ectopic overexpression of TIMP-1 in the brain of transgenic mice also reduces experimental metastasis to the brain [25]. In particular, MMP-2 and -9 are important for tumor invasion and angiogenesis. Thus, tumor metastasis can be inhibited by blocking synthesis and activity of MMP [26]. Strong anti-metastasis effects appeared in the reduction of MMPs and the increase of TIMPs via DCME in HCT-116 cells. From the results, DCME showed a strong anti-metastasis effect, and could be used as a part of functional food for cancer prevention.
In China, D. candidum, a rare Chinese medical material which can nourish yin, consists of various active substances. It can obviously increase many immune indexes, such as conversion rate of lymphocytes, improve syndrome of yin deficiency, and balance human organisms, thus protecting them from invasion of cancer [27]. The main efficacious ingredients in D. candidum were dendrobium polysaccharides, dendrobine, etc. According to some researchers, many soluble polysaccharides from D. candidum were immunopotentiators having strong anti-cancer bioactivity [28]. Polysaccharides from D. candidum could obviously increase the number of peripheral white blood cells and stimulate lymphocytes to produce migration inhibitory factors, both of which could efficiently eliminate side effects caused by immunosuppressor of cyclophosphamide (a commonly-used antineoplastic) [29]. In addition, they could also inhibit solid tumors, and, to some extent, improve conversion function of T-lymphocytes, NK activity, and levels of macrophage and hemolysin; although the amount of dendrobine in D. candidum was not very high, it is still very effective for its good quality [30]. D. candidum could efficiently inhibit lung cancer cells, atrophic gastritis, and diabetes. In experimental conditions, the inhibition rate could reach as high as over 70% [31]. Dendrobine was effective on anti-oxidation and anti-aging, and significantly increased SOD level and decreased LPO level. In addition, it could reduce the level of blood sugar, which was beneficial to treatment of diabetes [32]. If oxidation occurs in an organism, it is easy for the organism to become cancerous. However, anti-oxidation of D. candidum could effectively inhibit cancer at the early stages. In our other studies, we found that D. candidum consists of 11 dominant ingredients (dihydrogen resveratrol, dendromoniliside E, denbinobin, aduncin, adenosine, uridine, guanosine, defuscin, n-triacontyl cis-p-coumarate, hexadecanoic acid, and hentriacontane), most of which were effective on anti-cancer and health care [33]. Synthetic action of these ingredients might be the reason why D. candidum is effective as an anti-cancer agent. Many studies have reported on the effects of D. candidum on lung cancer, liver cancer, gastric cancer, esophageal cancer, and nasopharyngeal carcinoma [34,35]. However, no study on its effects on colon cancer has been reported. In the current study, the effects of D. candidum on colon cancer in vitro were studied using HCT-116 cells and in vivo using 26-M3.1 cells, both of which exerted beneficial effects. Stimulation of apoptosis in cancer cells and inhibition of their metastasis is the most important way to prevent tumor development. Results of the experiment showed that D. candidum could promote apoptosis in cancer cells and inhibit their metastasis in mice. The result may be achieved by some efficacious ingredients in D. candidum by improving the body's immunity and exerting immediate action on cancer cells.
In summary, we found that DCME has potent in vitro and in vivo anti-cancer activities, particularly for combating in vivo tumor metastasis. The scientific data proved the functional effects, and the results provided the scientific basis for development of DCME for further anticancer initiatives. The important active compounds resulting from D. candidum and combined actions of the compounds should be identified and evaluated in future studies, and investigation of the activities in humans is also needed.
Figures and Tables
Fig. 1
Exposure of HCT-116 human colon cancer cells to DCME induces apoptosis. (A) Appearance of apoptotic bodies in HCT-116 cells treated with different concentrations of DCME for 48 h. (B) Treatment with DCME for 48 h increased the number of apoptotic cells as measured by flow cytometry. The profile represents an increased sub-G1 population (apoptotic cells) and each point represents the mean ± SD of three independent experiments.
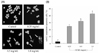
Fig. 2
Effects of DCME on the mRNA and protein expressions of Bax, Bcl-2, and caspases in HCT-116 human colon cancer cells. (A) RT-PCR; (B) Western blot.
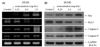
Fig. 3
Effects of DCME on the mRNA and protein expressions of NF-κB and IκB-α in HCT-116 human colon cancer cells. (A) RT-PCR; (B) Western blot.

Fig. 4
Effects of DCME on the mRNA and protein expressions of MMPs and TIMPs in HCT-116 human colon cancer cells. (A) RT-PCR; (B) Western blot.

Table 2
Inhibitory effects of DCME on metastasis of tumors produced by colon 26-M3.1 cells in Balb/c mice

References
1. Jones WE, Kuehnle AR, Arumuganathan K. Nuclear DNA content of 26 orchid (Orchidaceae) genera with emphasis on Dendrobium. Ann Bot. 1998; 82:189–194.

2. Shao BM, Xu W, Dai H, Tu P, Li Z, Gao XM. A study on the immune receptors for polysaccharides from the roots of Astragalus membranaceus, a Chinese medicinal herb. Biochem Biophys Res Commun. 2004; 320:1103–1111.

3. Shao H, Zhang LQ, Li JM, Wei RC. Advances in research of Dendrobium officinale. Chin Tradit Herb Drugs. 2004; 35:109–112.
6. Brew K, Dinakarpandian D, Nagase H. Tissue inhibitors of metalloproteinases: evolution, structure and function. Biochim Biophys Acta. 2000; 1477:267–283.

7. Bao LJ, Wang JH, Luo JP. Inhibitory effects of water extracts from four species of Dendrobiums on HelaS3 cells and HepG2 cells. J Anhui Agric Sci. 2008; 36:15968–15970.
8. Jung KO, Park SY, Park KY. Longer aging time increases the anticancer and antimetastatic properties of doenjang. Nutrition. 2006; 22:539–545.

9. van Meerloo J, Kaspers GJ, Cloos J. Cell sensitivity assays: the MTT assay. Methods Mol Biol. 2011; 731:237–245.

10. Harish Kumar G, Vidya Priyadarsini R, Vinothini G, Vidjaya Letchoumy P, Nagini S. The neem limonoids azadirachtin and nimbolide inhibit cell proliferation and induce apoptosis in an animal model of oral oncogenesis. Invest New Drugs. 2010; 28:392–401.

11. Malla R, Gopinath S, Alapati K, Gondi CS, Gujrati M, Dinh DH, Mohanam S, Rao JS. Downregulation of uPAR and cathepsin B induces apoptosis via regulation of Bcl-2 and Bax and inhibition of the PI3K/Akt pathway in gliomas. PLoS One. 2010; 5:e13731.

12. Li Y, Wang C, Wang F, Dong H, Guo S, Yang J, Xiao P. Chemical constituents of Dendrobium candidum. Zhongguo Zhong Yao Za Zhi. 2010; 35:1715–1719.
13. Li J, Li S, Huang D, Zhao X, Cai G. Advances in the of resources, constituents and pharmacological effect of Dendrobium officinale. Ke Ji Dao Bao. 2011; 29:74–79.
14. Milanezi F, Leitão D, Ricardo S, Augusto I, Schmitt F. Evaluation of HER2 in breast cancer: reality and expectations. Expert Opin Med Diagn. 2009; 3:607–620.

15. Chao DT, Korsmeyer SJ. BCL-2 family: regulators of cell death. Annu Rev Immunol. 1998; 16:395–419.

17. Kirsch DG, Doseff A, Chau BN, Lim DS, de Souza-Pinto NC, Hansford R, Kastan MB, Lazebnik YA, Hardwick JM. Caspase-3-dependent cleavage of Bcl-2 promotes release of cytochrome c. J Biol Chem. 1999; 274:21155–21161.

18. Delić R, Štefanović M. Optimal laboratory panel for predicting preeclampsia. J Matern Fetal Neonatal Med. 2010; 23:96–102.

19. Baeuerle PA. IkappaB-NF-kappaB structures: at the interface of inflammation control. Cell. 1998; 95:729–731.
20. Sánchez-Pérez I, Benitah SA, Martínez-Gomariz M, Lacal JC, Perona R. Cell stress and MEKK1-mediated c-Jun activation modulate NFkappaB activity and cell viability. Mol Biol Cell. 2002; 13:2933–2945.

21. Klein CA. Cancer. The metastasis cascade. Science. 2008; 321:1785–1787.
22. Ha ES, Hwang SH, Shin KS, Yu KW, Lee KH, Choi JS, Park WM, Yoon TJ. Anti-metastatic activity of glycoprotein fractionated from Acanthopanax senticosus, involvement of NK-cell and macrophage activation. Arch Pharm Res. 2004; 27:217–224.

23. Zucker S, Vacirca J. Role of matrix metalloproteinases (MMPs) in colorectal cancer. Cancer Metastasis Rev. 2004; 23:101–117.

24. Mysliwiec AG, Ornstein DL. Matrix metalloproteinases in colorectal cancer. Clin Colorectal Cancer. 2002; 1:208–219.

25. Krüger A, Sanchez-Sweatman OH, Martin DC, Fata JE, Ho AT, Orr FW, Rüther U, Khokha R. Host TIMP-1 overexpression confers resistance to experimental brain metastasis of a fibrosarcoma cell line. Oncogene. 1998; 16:2419–2423.

26. Shin DY, Kim GY, Kim JI, Yoon MK, Kwon TK, Lee SJ, Choi YW, Kang HS, Yoo YH, Choi YH. Anti-invasive activity of diallyl disulfide through tightening of tight junctions and inhibition of matrix metalloproteinase activities in LNCaP prostate cancer cells. Toxicol In Vitro. 2010; 24:1569–1576.

27. Wang Q, Sun P, Li G, Zhu K, Wang C, Zhao X. Inhibitory effects of Wall ex Lindl. on azoxymethane- and dextran sulfate sodium-induced colon carcinogenesis in C57BL/6 mice. Oncol Lett. 2014; 7:493–498.

28. Wanqian L, Bochu W, Chuanren D, Biao L. A method for isolating functional RNA from callus of Dendrobium candidum contented rich polysaccharides. Colloids Surf B Biointerfaces. 2005; 42:259–262.

29. Chen J, Qi H, Li JB, Yi YQ, Chen D, Hu XH, Wang ML, Sun XL, Wei XY. Experimental study on Dendrobium candidum polysaccharides on promotion of hair growth. Zhongguo Zhong Yao Za Zhi. 2014; 39:291–295.
30. Gao J, Jin R, Wu Y, Zhang H, Zhang D, Chang Y, Hu Z. Comparative study of tissue cultured Dendrobium protocorm with natural Dendrobium candidum on immunological function. Zhong Yao Cai. 2002; 25:487–489.
31. Chaotham C, Pongrakhananon V, Sritularak B, Chanvorachote P. A Bibenzyl from Dendrobium ellipsophyllum inhibits epithelial-to-mesenchymal transition and sensitizes lung cancer cells to anoikis. Anticancer Res. 2014; 34:1931–1938.
32. Deng MZ, Li TM. The effect of Dendrobium mixture on MDA, SOD, LPO and the immune function of aged rats. China J Chin Med. 2012; 27:58–59.
33. Li GJ, Sun P, Qian Y, Zhao X. Preventive effect of lung metastases of Dendrobium candidum Wall ex Lindl. in BALB/c mice bearing 26-M3.1 cells. Oncol Lett. Forthcoming 2014.
34. Chen J, Zhang LC, Xing YM, Wang YQ, Xing XK, Zhang DW, Liang HQ, Guo SX. Diversity and taxonomy of endophytic xylariaceous fungi from medicinal plants of Dendrobium (Orchidaceae). PLoS One. 2013; 8:e58268.

35. Prasad R, Koch B. Antitumor activity of ethanolic extract of Dendrobium formosum in T-cell lymphoma: an in vitro and in vivo study. Biomed Res Int. 2014; 2014:753451.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download