Introduction
Obesity is induced by the hypertrophy of adipocytes and the generation of new adipocytes from preadipocytes [1, 2]. Increased adiposity is considered to be one of the most important risk factors for arteriosclerosis, diabetes and hyperlipidemia, and is a major public health problem in many countries. Therefore, identifying molecular basis for controlling adipocytes has an implication for therapeutic modalities of obesity and obesity related metabolic consequences.
A number of CCAAT/enhancer-binding protein(C/EBP) family transcription factors, C/EBPα and β, and peroxisome proliferators-activated receptor γ (PPARγ) play critical roles in adipogenesis [3]. During adipogenesis, C/EBP β is rapidly induced by stimulation of hormone signaling and function as transcriptional regulators of C/EBPα- and PPARγ [4]. Adenosine monophosphate-activated protein kinase (AMPK) is also a possible molecular candidate of controlling adipocyte differentiation [5]. AMPK has emerged as an important target for controlling metabolic diseases such as type 2 diabetes(T2D), obesity and cancers and it is activated by several anti-diabetic drugs such as metformin and rosiglitazone [6]. AMPK is activated under ATP-depleting conditions and subsequently induces ATP-generation pathways for maintain cellular homeostasis [7-9]. Activation of AMPK stimulates the pathways that increases energy production, such as, glucose transport and fatty acid oxidation, and switches off pathways that consume energy, such as lipogenesis. It is well-established that AMPK regulates the expression of sterol regulatory element-binding proteins (SREBPs) and its target genes such as adipocyte fatty acid binding protein (aP2) and fatty acid synthase(FAS) [10,11].
Pear contains phenolic compounds including arbutin, catechin, epicatechin chlorogeic acid, caffeic acid and coumaric acid, which play a role as antioxidants [12]. Pear also contains edible fibers [13]. It was reported that phenolic compounds and edible fibers in pear prevented hyperglycemia and dislipidemia [14,15]. Pear pomace is the by-product produced during the preparation of pear juice, pear paste and pear makjeilli. Pear pomace contains useful substances, including phenolic compounds, organic acids and edible fibers. However, most of pear pomace is thrown out in the fields, causing environmental pollutions and only a small quantity of pear pomace is used as animal feed.
In the present study, we show that pear pomace water extract suppresses adipocyte differentiations and TG accumulations via inhibition of adipogenesis gene expression and activation of AMPK. We also observed the pear pomace water extract induced apoptosis of mature 3T3-L1 cells. Based on our results, we suggest that pear pomace may be used as a therapeutic agent for treating obesity and obesity-related disorders.
Materials and Methods
Preparation of pear pomace extracts
Pear pomace was prepared by water extraction. Briefly, 100g of pear pomace was soaked in 2000ml water for 24 hours at room temperature. The extract was filtered under reduced pressure, evaporated and freeze-dried. The yields obtained were 32.6%.
Cell culture
3T3-L1 mouse embryo fibroblasts were obtained from the American Type Culture Collection (Manassas, VA). Cells were cultured in Dulbecco's modified Eagle's medium (DMEM; Gibco, Gaithersburg), supplemented with penicillin 100 units/ml, streptomycin 100 µg/ml and 10% fetal calf serum, and incubated in a humidified incubator at 37℃ and 5% CO2.
MTT assay
The 3T3-L1 preadipocytes were seeded at a density of 1×104 cells/well in 96 well plates. The cells were then treated with pear pomace extracts at various concentrations for 24 hours. After completion of the treatment, the cells were incubated with an MTT solution (MTT: 2 mg/ml, Sigma-Aldrich, USA) for 4 hours at 37℃. The supernatants were aspirated, DMSO (Sigma-Aldrich, USA) were added to each well. After incubation for 15 minutes, absorbance was measured at 540 nm using a ELASA.
Differentiation of 3T3-L1 preadipocytes
To induce preadipocyte differentiation, 3T3-L1 preadipocytes were cultured until confluent. Two days after confluence (DO), the cells were stimulated to differentiation with DMEM containing 10% fetal bovine serum (FBS), 5 mg/ml insulin (Sigma, St. Louis, MO, USA), 0.5 mM isobutylmethylxanthine (IBMX: Sigma, St. Louis, MO, USA), and 1 µM dexamethasone (Sigma, St. Louis, MO, USA) for two days (D2). Cells were then maintained in 10% FBS DMEM containing 5 mg/ml insulin for another two days (D4), followed by culturing with 10% FBS DMEM medium for an additional 4 days (D8), at which more than 90% of cells were mature adipocytes with accumulated fat droplets. To examine the effect of pear pomace extract on adipogenesis, the extracts were dissolved in the differentiation medium.
Oil Red O staining
Oil Red O staining was performed on day 8. Cells were washed once or twice with phosphate-buffered saline (PBS) and fixed with 10% formalin for 30 minutes, and then cells were stained with Oil-Red O for 1 hour, Thereafter, the stained cells were washed twice with water.
After Oil Red O staining, lipid droplet cells were dissolved in isopropanol and quantified by ELISA (540nm), and were photographed by a phase-contrast microscope (Olympus CKX41) in combination with a digital camera (Canon) at 10 × magnification.
Immunoblotting
The mature 3T3-L1 adipocytes were washed once with cold PBS and scraped into lysis buffer (10mM Tris, 100mM NaCl, 1mM EDTA, 1 mM EGTA, 1 mM NaF, 20 mM Na4P2O7, 2 mM Na3VO4, 1% Triton × 100, 10% glycerol, 0.1% sodium dodecyl sulfate(SDS), 0.5% deoxycholate, 1mM PMSF, 5% protease inhibitor cocktail, and pH 7.4) for 10 minutes. The cell mixture was centrifuged at 13,000 rpm for 20 minutes at 4℃, the supernatant was collected as the lysate, and the protein concentration was determined by a Bradford protein assay (Sigma, St. Louis, MO, USA). The protein (25 µg) was mixed with 5 sample buffer containing 60 mM Tris-HCl (pH 6.8), 25% glycerol, 2% SDS, 10% bromophenol, and 5% 2-mercaptoethanol, heated at 95℃ for 10 minutes, and separated by SDS-polyacrylamide gel electrophoresis. After electrophoresis, the proteins were transferred onto a nitrocellulose membrane using a transblot chamber with Tris buffer (0.025 M Tris-HCl, 0.192 M glycine, and 20% MeOH). The membrane was blocked for 1 hour at 4℃ with 5% nonfat milk in TBS-T buffer containing 10 mM Tris-HCl (pH 6.8), 100 mM NaCl, and 0.1% Tween 20. The membranes were then incubated overnight with primary antibodies [PPARγ 1:1000 (Cell Signaling Technology, Beverly, MA, USA), C/EBP α (Santa Cruz Biotechnology, Santa Cruz, CA, USA) 1:250, p-AMPK 1:1000 (Cell Signaling Technology), FAS 1:1000 (Cell Signaling Technology), SREBP1-c (Santa Cruz Biotechnology) 1:250 and β-actin (Santa Cruz Biotechnology) 1:1,000]. After washing with TBS-T buffer, the membranes were incubated with horseradish peroxidase conjugated secondary antibody [goat anti-rabbit or mouse anti-goat (1:2000)] for 1 hour at room temperature. Bands were detected by chemiluminescent substrate (IMGENEX,San Diego, CA), and the protein expression was quantified using UVP (imaging system for chemiluminescent Western blot, Upland, CA) and VisionWorksTMLS (Analysis Software, Upland, CA) using UVP (imaging system for chemiluminescent Western blot, Upland, CA) and VisionWorksTMLS (Analysis Software, Upland, CA).
Apoptosis
For the assessments of apoptosis, the Muse™ Annexin V & Dead Cell Kit was used. For mature adipocytes, cells were seeded and grown to maturation as described above. The adipocytes were incubated with either PBS or pear pomace extract for 24 hours and 48 hours. Thereafter, treatment medium was removed and washed with PBS, and collected using trypsin-EDTA. The harvested cells were centrifuged at 1,400rpm for 5 minutes and re-suspended with complete medium. Muse™ Annexin V & Dead Cell reagent (100 µl) was added to each to re-suspended cell and incubated for 20 minutes. At the end of 90 minutes, the re-suspended cell was assayed by Muse Cell Analyzer. The Annexin V-PE(-) and dead cell marker(-) population was regarded as viable cells, not undergoing detectable apoptosis, while the Annexin V-PE(+) and dead cell marker(-) population was taken as a measure of early apoptosis, the Annexin V-PE(+) and dead cell marker(+) as the late stages of apoptosis or dead by apoptotic mechanism, the Annexin V-PE(-)and dead cell marker(+) as necrosis but nor through the apoptotic pathway.
Results
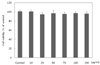
Effect of pear pomace water extract on cell viability
3T3-L1 cells were exposed to various concentration of pear pomace water extracts in dose dependent manner, and intracellular toxicity was measured by MTT assay. As shown Fig. 1, water extract of pear pomace at concentration of 250 µg/ml showed no significant effects on viability after 24 treatments.
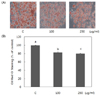
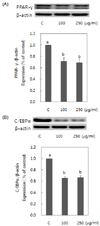
Pear pomace water extract inhibits 3T3-L1 adipocyte differentiation and the expression level of key transcriptional regulators of differentiation
The effects of pear pomace on lipid accumulation was examined by Oil Red O staining of 3T3-L1 adipocytes. There was no significant difference compared with control when we treated cells with pomace extract at a concentration of 50 µg/ml (data were not shown). So, cells were treated with 100 and 250 µg/ml for further study. Pear pomace water extracts significantly reduced lipid accumulation, as indicated by decreased Oil Red O staining (P < 0.05) (Fig. 2A). Triglyceride contents in 3T3-L1 adipocytes decreased in a dose dependent manner (Fig. 2B). We performed western blotting analysis to determine whether pear pomace inhibited adipocyte differentiation by negatively regulating the expression of C/EBPα and PPARγ, the key transcriptional regulators. Pear pomace water extracts significantly inhibited the protein expression of C/EBPα and PPARγ (P < 0.05) (Fig. 3A & B).
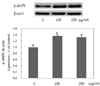
Pear pomace water extract activates AMPK to p-AMPK
AMP-activated protein kinase (AMPK) activation was determined by Western blotting analysis of p-AMPK. As shown in Fig. 4, the AMPK was significantly activated by pear pomace extracts.
Pear pomace water extract inhibits the expression level of terminal markers of adipogenesis
We examined the protein expression levels of FAS and SREBP to determine the effects of pear pomace on the expression of C/EBPα and PPARγ target genes (Fig. 5). Pear pomace water extract inhibited the expression of terminal markers of adipogenesis such as fatty acid synthase (FAS) and SREBP (P < 0.05).
Pear pomace water extracts increases apoptosis of 3T3-L1 cells
Pear pomace water extracts increased apoptosis of 3T3-L1 cells treated for 24 and 48 hours (Fig. 6). Cells in lower-right quadrant [cells in the early stages of apoptosis : Annexin V-PE(+) and Dead Cell Marker(-)] and upper-right quadrant [cells in the late stages of apoptosis or dead by apoptotic mechanism : Annexin V-PE(+) and Dead Cell Marker(+)] were significantly increased by pear pomace (Fig. 6(B)).
Discussion
In the present study, we demonstrated that pear pomace inhibited adipogenesis and induced adipocyte apoptosis using the 3T3-L1cells. Adipogenesis is the process of preadipocyte differentiation to mature adipocyte, accompanied by coordinated changes in cell morphology, gene expression and hormone sensitivity [16]. The 3T3-L1 cell line has been used for identifying key molecular markers, transcription factors and various interactions that are required for adipogenesis [17-19], and is thus very useful to rapidly screen and assess the adipogenic potential of various agents.
In this study, we observed that pear pomace water extract significantly inhibited 3T3-L1 adipogenesis and fat accumulations (Fig. 2). Adipogenesis is regulated by several transcriptional factors, including PPARs and C/EBPs [10,11]. Pear pomace water extract significantly inhibited the expression levels of C/EBPα and PPARγ, two master regulators of adipogenesis (Fig. 3a, b). C/EBPα and PPARγ play a role in the initiation of adipocyte differentiation and induce the synthesis of various adipogenic genes [1]. Therefore, our results indicate that pear pomace water extract might suppress 3T3-L1 adipocyte differentiations via inhibiting the expression of adipogenesis-related transcription factors.
Possible mechanism of action for pear pomace induced inhibition of adipogenesis could involve the activation of AMP-activated protein kinase (AMPK). Activation of AMPK by phosphorylation has potential for reversing metabolic abnormalities associated with type 2 diabetes mellitus and thereby, AMPK has been a target for the discovery of drugs with potential efficacy for type 2 diabetes mellitus and obesity [12]. In the present study, pear pomace increased expressions of p-AMPK, the activated form of AMPK (Fig. 4). Since AMPK deactivates SREBP-1c which targets genes involved in fatty acid and triglyceride synthesis [20,21], and thereby inhibits lipogenesis [22], we examined the effect of pear pomace water extract on the expression of SREBP-1c protein. In our study, pear pomace water extract suppressed the expression of SREBP-1c protein (Fig. 5). Tang et al. [20] reported that betulin, a SREBP inhibitor, showed beneficial effects in vivo including prevention of diet-induced obesity and improvements of lipid profile and insulin resistance. They suggested that SREBP pathway can be a potential therapeutic target to treat obesity related metabolic diseases. The inhibitions of SREBP-1c mRNA expression were accompanied by a sharp reduction in the mRNA expression of SREBP-1c target genes, such as FAS and ACC [16]. We examined the effect of pear pomace water extract on the expression of FAS protein and observed pear pomace water extract to suppress the expression of FAS. Therefore, we can conclude that pear pomace can inhibit the differentiation of 3T3-L1 and accumulation of TG via inhibition of the adipogenesis-related transcription factor expression including activation of AMPK signaling.
The amount of adipose tissue mass can be decreased by deletions of adipocytes via apoptosis as well as by inhibitions of adipogenesis. Previous studies revealed that some anti-obesity agents caused induction of apoptosis as well as inhibition of adipogenesis of 3T3-L1 cells [23-25]. Therefore, we examined whether pear pomace has apoptotic activity in mature adipocytes or not. Cell apoptosis is important for destruction of undesired cells during development and homeostasis of multicellular organisms and is charaterised by distinct morphological changes [23]. It was reported that the induction of apoptosis by activated AMPK was observed in the AMPK over-expressed conditions of various cells [26,27]. Park et al. [23] showed that AMPK is necessary for the apoptosis of mature adipocyte by selenium and resveratrol. In the present study, the apoptosis of mature adipocyte was induced by pear pomace water extract. Based on the relationship of AMPK activation and apoptosis, our result shows that pear pomace activates AMPK and induces apoptosis of mature adipocytes.
Our study clearly identified that pear pomace water extract inhibited adipogenesis and resulted in apoptosis in adipocytes. Although, further study with AMPK inhibitor need to be done to confirm the activation of AMPK signaling as the mechanism for antiadipogenic and proapoptotic effects of pear pomace, we can carefully suggest that the mechanism of action for pear pomace induced antiadipogenic and proapoptic effects might involve the activation of AMPK signaling. Thus, pear pomace may prove to be a valuable mutual product in the treatment of obesity and obesity-related disorders.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download