Abstract
BACKGROUD/OBEJECTIVES
Arsenic, which causes human carcinogenicity, is ubiquitous in the environment. This study was designed to evaluate modulation of arsenic induced cancer by resveratrol, a phytoalexin found in vegetal dietary sources that has antioxidant and chemopreventive properties, in arsenic trioxide (As2O3)-induced Male Wistar rats.
MATERIALS/METHODS
Adult rats received 3 mg/kg As2O3 (intravenous injection, iv.) on alternate days for 4 days. Resveratrol (8 mg/kg) was administered (iv.) 1 h before As2O3 treatment. The plasma and homogenization enzymes associated with oxidative stress of rat kidneys were measured, the kidneys were examined histologically and trace element contents were assessed.
RESULTS
Rats treated with As2O3 had significantly higher oxidative stress and kidney arsenic accumulation; however, pretreatment with resveratrol reversed these changes. In addition, prior to treatment with resveratrol resulted in lower blood urea nitrogen, creatinine and insignificant renal tubular epithelial cell necrosis. Furthermore, the presence of resveratrol preserved the selenium content (0.805 ± 0.059 µg/g) of kidneys in rats treated with As2O3. However, resveratrol had no effect on zinc level in the kidney relative to As2O3-treated groups.
Humans are commonly exposed to arsenic, which is incorporated into food, drinking water, soil, dust, smoke and air [1,2]. Arsenic is known to be a potent carcinogen that can induce the formation of various types of solid tumors, including lung, prostate, bladder, renal, and skin cancers, as well as other malignancies via inhibition of oxidative phosphorylation, interference with cell signaling by binding to hormone receptors, or generation of reactive oxygen species (ROS) [3,4]. Conversely, arsenic has been used for the treatment of various diseases since 400 BC, and it is currently employed as an effective chemotherapeutic agent for the treatment of certain human cancers, such as acute promyelocytic leukemia (APL), non-APL acutemyeloid leukemia cells, chronic myeloid leukemia cells, and some solid tumor cells. It is well known that the kidney is a primary target for the toxic effects of arsenic, as evidenced by clinical manifestations and biochemical alterations [5]. Unfortunately, patients being treated for APL also suffer from nephrotoxicity. Among several mechanisms, oxidative stress is a relatively common cause of arsenic toxicity in kidneys [2,5]. Studies have shown that natural antioxidant components could lower the risk of cardiovascular diseases and several cancers and provide double protection from this disaster [6,7].
Resveratrol is a phytoalexin present in at least 72 plant species, many of which are consumed by humans, such as mulberries, peanuts, and grapes [8]. Numerous reports indicate that resveratrol has anti-inflammatory, antiplatelet, antioxidant, and anti-carcinogenic effects, as well as the ability to attenuate chemical compound-induced kidney injury [8,9]. However, it remains uncertain whether resveratrol can protect against arsenic-induced nephrotoxicity and its potential mechanism. Therefore, in this study, we investigated resveratrol, a novel option to protect arsenic trioxide (As2O3)-induced nephrotoxicity in rats.
As2O3 was purchased from Harbin Yida (Harbin, China). Resveratrol (purity, > 99%) was purchased from Sigma-Aldrich (St. Louis, MO, USA). Commercial assay kits for malondialdehyde (MDA), superoxide dismutase (SOD), glutathione peroxidase (GPx), ROS, total sulfhydryl (-SH), and to determine the total glutathione (T-GSH) to oxidized glutathione (GSSG) ratio (T-GSH/GSSG) were bought from the Nanjing Jiancheng Bioengineering Institute (Nanjing, China).
Male Wistar rats, aged 6-8 weeks, were obtained from the Experimental Animal Centre of Harbin Medical University (Harbin, China). The rats were grouped and housed in polyacrylic cages with not more than four animals per cage under controlled conditions of 25 ± 2℃, 50 ± 15% relative humidity and normal photoperiod (12 h dark and light), during which time they were provided standard rodent chow (Table 1) and water ad libitum. The rats were allowed to acclimatize to the laboratory environment for 7 days prior to the study. All experiments were approved by the Ethics Committee on the Use and Care of Animals, Northeast Agricultural University (Harbin, China) with the following reference number: NEAU2011100145.
Thirty-two rats were randomly divided into four groups: control, As2O3-treated, As2O3 + resveratrol, and resveratrol-treated. In the As2O3 group, rats were treated with As2O3 (3 mg/kg); in the As2O3 + resveratrol group, rats were given resveratrol (8 mg/kg) 1 h before As2O3 administration; the resveratrol group received 8 mg/kg body weight resveratrol. An equal amount of 0.9% normal saline was administered as a vehicle to control rats. All treatments were administered via the caudal vein on alternate days for 4 days (i.e., days 1, 3, 5, and 7) and measurements were made on the 8th day.
On the 8th day, rats were given ether anesthesia and euthanized. Blood samples were then collected by puncturing the retro-orbital venous sinus. Samples were centrifuged (4,000 × g for 20 min, 20℃) and serum was prepared. Blood urea nitrogen (BUN) and creatinine (CRE) levels were assayed using UniCel DxC800 Synchron (Bekman, USA). Additionally, kidneys were removed from the experimental rats at 4℃ and small representative tissue slices were taken for histopathological examinations. A portion of the kidney was also used to estimate the tissue arsenic, zinc and selenium concentrations. The remainder of the kidney was washed with ice-cold saline and homogenized in 4 volumes of ice-cold 0.1M phosphate buffer (pH 7.4) containing 0.15 M potassium chloride. The homogenate was then centrifuged at 10,000×g at 4℃ for 10min.
SOD and GPx activities, the content of MDA, ROS, and -SH and the T-GSH/GSSG ratio were measured using assay kits from Nanjing Jiancheng Bioengineering Institute (Nanjing, China) according to the manufacturer's protocol.
Kidney samples were fixed (10% formalin for 24 h, 37℃), dehydrated, embedded in paraffin and cut into 5µm thick sections. The sections were then stained with hematoxylin and eosin, after which they were examined under a light microscope (BX-FM; Olympus Corp, Tokyo, Japan). A minimum of 10 fields for each kidney slide were examined and changes were scored as either none (-), mild (+), moderate (++) or severe (+++) damage.
Arsenic contents in the kidney tissues of all rats were analyzed according to the method described Csanaky and Gregus [10]. Briefly, a representative sample of kidney was digested three times with a mixture of deionized water, nitric acid (HNO3) and hydrogen peroxide (H2O2) until almost dry, after which the residual mass was dissolved in 1% HNO3. The arsenic content of the solution was then estimated using a hydride generation system within an atomic fluorescence spectrometry system.
The concentrations of zinc in kidney tissues were measured with a flame atomic absorption spectrophotometer using standards for zinc for comparison and analyzed according to the method described by Kucukatay et al. [11]. Blank and standard solutions were used for calibration of the atomic absorption spectrophotometer.
The selenium content in the tissues was estimated using a method previously described by Entwisle and Hearn [12]. The assay is based on the principle that the selenium contained in samples is converted to selenous acid in response to acid digestion. The reaction between selenous acid and aromatic-o-diamines leads to the formation of 4, 5-benzopiazselenol, which displays a brilliant lime-green fluorescence when excited at 366 nm in cyclohexane and measured by fluorescence spectrometry.
Statistical analyses were conducted using the SPSS19.0 computer program (SPSS, Chicago, IL, USA). One-way analysis of variance (ANOVA) followed by Dunnett's test was applied to calculate the statistical significance between various groups. In all cases, a P < 0.05 was considered significant.
The SOD and GPx activities, MDA and ROS concentrations, and T-GSH/GSSG ratio in kidneys from all groups are shown in Fig. 1. In the As2O3-treated group, the MDA and ROS content in the kidney were far higher than in the control group, but the activities of SOD and GPx were lower. However, pretreatment with resveratrol markedly reversed these alterations. Furthermore, the T-GSH/GSSG ratio (4.64 ± 0.48) in the As2O3-treated group was significantly lower than that of the control group (6.36 ± 0.56). Conversely, resveratrol pre-administration (5.82 ± 0.47) significantly (P < 0.05) normalized content in the kidney (Fig. 1E).
The BUN (15.47 ± 1.91) and CRE (44.14 ± 1.90) levels were significantly increased following 4 days of alternating arsenic exposure (Fig. 2A); however, the administration of resveratrol led to a significant improvement in this value (11.86 ± 1.63; 34.40 ± 2.15, P < 0.05). The toxic effects of As2O3 were also confirmed by the detection of morphological alterations in kidney slices (Fig. 2).
The histopathological changes were graded and summarized in Table 2. The control group showed no morphological changes. In contrast, the kidneys of As2O3-treated rats showed cortex edema, tubular cell swelling, interstitial edema, glomeruli dilation and hyperemia, pyknotic nuclei and severe necrosis, and denudation of the tubular cells. Treatment with resveratrol attenuated renal morphological alterations (Fig. 2), which showed moderate glomeruli dilation, slight tubular cell swelling and edema.
Treatment with resveratrol alone did not induce a significant increase in -SH concentration relative to the control group. However, a single dose of As2O3 resulted in a significant 45.3% decrease in -SH level when compared with the control group. Similarly, treatment with resveratrol resulted in complete reversal of the As2O3-induced decrease in -SH level (Fig. 3).
Fig. 4 shows the effects of As2O3 on total arsenic, zinc and selenium concentration in kidney tissues from resveratrol-supplemented rats. Rats treated with As2O3 showed a significant 317.8% increase in total arsenic level in kidney tissues relative to the control group, while administration of resveratrol for 4 alternate days resulted in a 53% decrease when compared with the As2O3-treated group. In addition, to evaluate whether pre-treatment with resveratrol modulated the essential elements, we assessed the zinc and selenium levels in kidneys. Resveratrol had a markedly protective effect in the selenium level of kidneys during arsenic exposure, whereas treatment with or without resveratrol had no beneficial effect on zinc concentration (Fig. 4).
In several studies, small doses (0.02-8 mg/kg) of resveratrol administered prophylactically reduced or prevented the development of tumors in rats given different carcinogens [13,14]. Another small study found that a single dose of up to 5 g of trans-resveratrol caused no serious adverse effects in healthy volunteers [15]. In addition, our colleagues demonstrated that resveratrol (8 mg/kg, iv.) protected against arsenic-induced hepatotoxicity [16]. This dose of resveratrol (< 8 mg/kg) did not show a protective effect against As2O3-induced nephrotoxicity in a pre-experiment. Therefore, the dose of resveratrol in our study was 8 mg/kg administered iv.
Arsenic toxicity is associated with its reactivity and generation of ROS and sulfur containing compounds [2,17]. The kidney is highly vulnerable to damage caused by ROS, which are involved in the pathogenic mechanism of conditions [18]. Our study also showed that, after arsenic exposure, ROS-induced oxidative damage increased MDA and GSSH production in the kidney, which is consistent with the results of previous studies [18]. However, the intrinsic antioxidant defenses of cells play a crucial role in the elimination of free radicals, including enzymatic antioxidant and non-enzymatic systems [19]. Both SOD and GPx are important endogenous antioxidant enzymes that destroyed the superoxide anion radical through dismutation and generated H2O2. In addition, GSH is an important non-protein compound-containing thiol group associated with the maintenance of redox homoeostasis [16,20]. Maiti and Chatterjee [20] reported that GSH also stimulated the arsenic detoxification processes by modulating arsenic methylation and increasing hepatobiliary excretion of arsenic. Resveratrol is a naturally occurring stilbene, a potent free radical scavenger and may also stimulate endogenous antioxidant enzyme activities [21,22]. As expected, As2O3 treatment resulted in significant nephrotoxicity in rats, and pre-treatment with resveratrol attenuated or prevented the oxidative stress, thereby mitigating the subsequent renal damage. These data indicated that resveratrol could markedly renew the activities of those antioxidant enzymes in the kidneys of As2O3-treated rats and at least partly attenuate oxidative stress by increasing the SOD, GPx and GSH/GSSG ratio as well as decreasing MDA and ROS levels in As2O3-treated rat kidney, thereby ameliorating the defense capacity of rats.
To further understand the protective mechanism of resveratrol against As2O3-induced nephrotoxicity, we analyzed the content of -SH, zinc and selenium. Toxicity due to trivalent arsenic might be attributed to direct binding with the -SH group [3]. In addition, zinc and selenium are essential for the activity of many enzymes that defend biological systems against damage caused by activated oxygen [10,11]. Recent advances have shown that supplemental sulfhydryl (thiol), zinc or selenium can act as complimentary chelator (adjuvant) agents, increasing the efficacy of known chelators, or by acting independently [12, 23,24]. Therefore, it is likely that maintenance of -SH, zinc and selenium content can attenuate toxicity. In the present study, we demonstrated that treatment of rats with resveratrol preserved the depletion of -SH content after arsenic exposure, indicating that resveratrol acted as an alternative agent or agonist of -SH directly or indirectly. Although the concentration of selenium in the kidney is very low, the antioxidant function is apparent due to a component of the antioxidant enzyme, GPx. Consequently, the presence of resveratrol restored the depletion of selenium rats with As2O3. However, arsenic exposure resulted in no difference in the zinc content between rats treated with and without resveratrol. These findings indicate that, the depleted renal zinc concentration remained insensitive after arsenic absorption, regardless of the presence of resveratrol. A second finding of interest is our observation that resveratrol affected arsenic metabolism and facilitated arsenic efflux. GSH reportedly plays a vital role in arsenic metabolism via involvement in arsenic reduction and efflux [16]. Moreover, Flora et al. [25] suggested that arsenic increased hepatobiliary transport of selenium and selenite, which led to increased biliary excretion of arsenic. Therefore, we suggest that arsenic efflux was facilitated by maintenance of the GSH/GSSG ratio and selenium content, as might be achieved with resveratrol supplementation.
In conclusion, the protective actions of resveratrol against As2O3 are believed to stem from its direct free radical scavenging, indirect antioxidant activities, and/or the maintenance of -SH and selenium content. However, we cannot rule out the possibility that the observed results are due to a positive association between resveratrol and several families of multispecific drug transporters, in evidence, nephrotoxicity will occur only after being transported into the renal cells [26] and metabolomics [27]. Therefore, additional evidence should be provided and extensive clinical trials should be conducted to confirm the beneficial effects of resveratrol in humans and other animals.
Figures and Tables
Fig. 1
Effect of resveratrol on ROS level and activities of antioxidant enzymes in kidneys of As2O3-treated rats. (A) Level of MDA; (B) ROS Level; (C) SOD activity; (D) GPx activity; (E) T-GSH/GSSG ratio. Each value is expressed as the mean ± SEM (n = 8). *P < 0.05 when compared with the control group; #P < 0.05 when compared with the As2O3-treated group. MDA, malondialdehyde; ROS, reactive oxygen species; SOD, superoxide dismutase; GPx, glutathione peroxidase; T-GSH/GSSG, total glutathione to oxidized glutathione ratio.

Fig. 2
Functional markers and morphological evaluation of rat kidneys. (A) BUN and CRE content in serum; (B) control rats; (C) As2O3-treated rats; (D) As2O3-treated rats with resveratrol. All values are expressed as the mean ± SEM (n = 8). *P < 0.05 when compared with the control group; #P < 0.05 when compared with the As2O3-treated group. Original magnification, 10×20
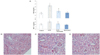
Fig. 3
Effect of resveratrol on arsenic induced renal -SH content in rat. All values are expressed as the mean ± SEM. (n = 8). *P < 0.05 when compared with the control group; #P < 0.05 when compared with the As2O3-treated group. -SH, total sulfhydryl.

Fig. 4
Effect resveratrol on arsenic, zinc and selenium concentration (µg/g for tissue) in exposed rats. (A) arsenic and zinc concentration in kidney; (B) selenium concentration in kidney. Values are expressed as the mean ± SEM. (n = 8). *P < 0.05 when compared with the control group; #P < 0.05 when compared with the As2O3-treated group.

ACKNOWLEDGEMENTS
This study was supported in part by the National Science Foundation Committee of China (31101868), Heilongjiang Province Foundation for Young Scholars (QC2010057), Chinese Postdoctoral.
Science Foundation (20100481040) and the Program for New Century Excellent Talent in Heilongjiang Provincial University (1253-NCET-007). The authors are grateful to Dr. Mutoko Mukai, Population Medicine and Diagnostic Sciences/Toxicology, College of Veterinary Medicine, Cornell University, New York, USA for guidance.
References
1. Yu M, Xue J, Li Y, Zhang W, Ma D, Liu L, Zhang Z. Resveratrol protects against arsenic trioxide-induced nephrotoxicity by facilitating arsenic metabolism and decreasing oxidative stress. Arch Toxicol. 2013; 87:1025–1035.

2. Hughes MF, Beck BD, Chen Y, Lewis AS, Thomas DJ. Arsenic exposure and toxicology: a historical perspective. Toxicol Sci. 2011; 123:305–332.

3. Singh S, Mulchandani A, Chen W. Highly selective and rapid arsenic removal by metabolically engineered Escherichia coli cells expressing fucus vesiculosus metallothionein. Appl Environ Microbiol. 2008; 74:2924–2927.

4. Wang L, Kou MC, Weng CY, Hu LW, Wang YJ, Wu MJ. Arsenic modulates heme oxygenase-1, interleukin-6, and vascular endothelial growth factor expression in endothelial cells: roles of ROS, NF-κB, and MAPK pathways. Arch Toxicol. 2012; 86:879–896.

5. Li Z, Piao F, Liu S, Wang Y, Qu S. Subchronic exposure to arsenic trioxide-induced oxidative DNA damage in kidney tissue of mice. Exp Toxicol Pathol. 2010; 62:543–547.

6. Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006; 5:493–506.

7. Nandi D, Patra RC, Swarup D. Effect of cysteine, methionine, ascorbic acid and thiamine on arsenic-induced oxidative stress and biochemical alterations in rats. Toxicology. 2005; 211:26–35.

8. Dudka J, Gieroba R, Korga A, Burdan F, Matysiak W, Jodlowska-Jedrych B, Mandziuk S, Korobowicz E, Murias M. Different effects of resveratrol on dose-related Doxorubicin-induced heart and liver toxicity. Evid Based Complement Alternat Med. 2012; 2012:606183.

9. Gülçin İ. Antioxidant activity of food constituents: an overview. Arch Toxicol. 2012; 86:345–391.

10. Csanaky I, Gregus Z. Effect of selenite on the disposition of arsenate and arsenite in rats. Toxicology. 2003; 186:33–50.

11. Kucukatay V, Turgut S, Kocamaz E, Emmungil G, Bor-Kucukatay M, Turgut G, Akca H, Bagci H. Effect of sulfite exposure on zinc, iron, and copper levels in rat liver and kidney tissues. Biol Trace Elem Res. 2006; 114:185–195.

12. Entwisle J, Hearn R. Development of an accurate procedure for the determination of arsenic in fish tissues of marine origin by inductively coupled plasma mass spectrometry. Spectrochim Acta Part B At Spectrosc. 2006; 61:438–443.

13. Athar M, Back JH, Tang X, Kim KH, Kopelovich L, Bickers DR, Kim AL. Resveratrol: a review of preclinical studies for human cancer prevention. Toxicol Appl Pharmacol. 2007; 224:274–283.

14. Scott E, Steward WP, Gescher AJ, Brown K. Resveratrol in human cancer chemoprevention--choosing the 'right' dose. Mol Nutr Food Res. 2012; 56:7–13.
15. Boocock DJ, Faust GE, Patel KR, Schinas AM, Brown VA, Ducharme MP, Booth TD, Crowell JA, Perloff M, Gescher AJ, Steward WP, Brenner DE. Phase I dose escalation pharmacokinetic study in healthy volunteers of resveratrol, a potential cancer chemopreventive agent. Cancer Epidemiol Biomarkers Prev. 2007; 16:1246–1252.

16. Zhang W, Xue J, Ge M, Yu M, Liu L, Zhang Z. Resveratrol attenuates hepatotoxicity of rats exposed to arsenic trioxide. Food Chem Toxicol. 2013; 51:87–92.

17. Lu KH, Lee HJ, Huang ML, Lai SC, Ho YL, Chang YS, Chi CW. Synergistic apoptosis-inducing antileukemic effects of arsenic trioxide and mucuna macrocarpa stem extract in human leukemic cells via a reactive oxygen species-dependent mechanism. Evid Based Complement Alternat Med. 2012; 2012:921430.
18. Rodrigo R, Rivera G. Renal damage mediated by oxidative stress: a hypothesis of protective effects of red wine. Free Radic Biol Med. 2002; 33:409–422.

19. Han JY, Ahn SY, Oh EH, Nam SY, Hong JT, Oh KW, Lee MK. Red ginseng extract attenuates kainate-induced excitotoxicity by antioxidative effects. Evid Based Complement Alternat Med. 2012; 2012:479016.

20. Maiti S, Chatterjee AK. Effects on levels of glutathione and some related enzymes in tissues after an acute arsenic exposure in rats and their relationship to dietary protein deficiency. Arch Toxicol. 2001; 75:531–537.

21. Kang NE, Ha AW, Kim JY, Kim WK. Resveratrol inhibits the protein expression of transcription factors related adipocyte differentiation and the activity of matrix metalloproteinase in mouse fibroblast 3T3-L1 preadipocytes. Nutr Res Pract. 2012; 6:499–504.

22. Lee HS, Ha AW, Kim WK. Effect of resveratrol on the metastasis of 4T1 mouse breast cancer cells in vitro and in vivo. Nutr Res Pract. 2012; 6:294–300.

23. Misbahuddin M, Islam AZ, Khandker S, Ifthaker Al M, Islam N, Anjumanara . Efficacy of spirulina extract plus zinc in patients of chronic arsenic poisoning: a randomized placebo-controlled study. Clin Toxicol (Phila). 2006; 44:135–141.

24. Pal S, Chatterjee AK. Protective effect of N-acetylcysteine against arsenic-induced depletion in vivo of carbohydrate. Drug Chem Toxicol. 2004; 27:179–189.

25. Flora SJ, Kannan GM, Kumar P. Selenium effects on gallium arsenide induced biochemical and immunotoxicological changes in rats. Chem Biol Interact. 1999; 122:1–13.

26. Anzai N, Endou H. World Congress on Alternatives and Animal Use in the Life Sciences. Renal drug transporters and nephrotoxicity. In : Review Progress Made toward the 3Rs : Proceedings / 6th World Congress on Alternatives & Animal Use in the Life Sciences; Tokyo: Japanese Society for Alternatives to Animal Experiments;2008. p. 447–452.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download