Abstract
Obesity is an epidemic disease characterized by an increased inflammatory state and chronic oxidative stress with high levels of pro-inflammatory cytokines and lipid peroxidation. Moreover, obesity alters cholesterol metabolism with increases in low-density lipoprotein (LDL) cholesterols and triglycerides and decreases in high-density lipoprotein (HDL) cholesterols. It has been shown that mulberry leaf and fruit ameliorated hyperglycemic and hyperlipidemic conditions in obese and diabetic subjects. We hypothesized that supplementation with mulberry leaf combined with mulberry fruit (MLFE) ameliorate cholesterol transfer proteins accompanied by reduction of oxidative stress in the high fat diet induced obesity. Mice were fed control diet (CON) or high fat diet (HF) for 9 weeks. After obesity was induced, the mice were administered either the HF or the HF with combination of equal amount of mulberry leaf and fruit extract (MLFE) at 500mg/kg/day by gavage for 12 weeks. MLFE treatment ameliorated HF induced oxidative stress demonstrated by 4-hydroxynonenal (4-HNE) and modulated the expression of 2 key proteins involved in cholesterol transfer such as scavenger receptor class B type 1 (SR-B1) and ATP-binding cassette transporter A1 (ABCA1) in the HF treated animals. This effect was mainly noted in liver tissue rather than in cutaneous tissue. Collectively, this study demonstrated that MLFE treatment has beneficial effects on the modulation of high fat diet-induced oxidative stress and on the regulation of cholesterol transporters. These results suggest that MLFE might be a beneficial substance for conventional therapies to treat obesity and its complications.
Obesity is an epidemic condition, which has increased prevalence in recent decades due to the changes in lifestyles including diets [1,2]. Obesity is considered as a risk factor for several diseases such as hypertension, type 2 diabetes, dyslipidemia, renal disease, cardiovascular disease, and cancer [3]. Oxidative stress has been regarded as an important factor in obesity [4]. Oxidative stress is described as the imbalance between pro-oxidants and anti-oxidants, resulting from increased production of reactive oxygen species (ROS) that exceeds the ability of antioxidant defense systems [5]. Consequentially, the increase of obesity-associated oxidative stress leads to biochemical changes including lipid peroxidation, DNA damage, and the change of enzymatic activity [6-8]. 4-hydroxynonenal (4-HNE) is recognized as an important biomarker of lipid peroxidation in a state of obesity-induced oxidative stress [9]. The concentration of 4-HNE as a product of oxidative stress was increased in obese subjects compared to healthy subjects [10], and in particular, the overexpression of 4-HNE level causes fat accumulation that worsens the state of obesity, resulting in metabolic syndrome [9].
In addition, abnormalities in both lipids and lipoproteins such as increases in low-density lipoprotein (LDL) cholesterols and triglycerides and decreases in high-density lipoprotein (HDL) cholesterols are considered to be the crucial pathway in obesity [11]. Moreover, pro-inflammatory markers such as TNF-α and CRP may down-regulate the apo A-1 gene expression that could correlate with one of the reasons why HDL cholesterol levels are lower in the obesity. The low levels of HDL cholesterol could be a consequence of increased serum inflammatory molecules such as TNF-α and CRP. From this point of view, the scavenger receptor class B type 1 (SR-B1), which is defined as a cell surface HDL receptor, could be a representative bio-marker to understand HDL metabolism along with the several lipoproteins in obesity-related change to maintain the lipid and lipoprotein homeostasis.
Accordingly, the increase in SR-B1 expression is correlated with decreased plasma levels of HDL cholesterol [12] and hepatic SR-B1 expression can be modulated by various factors including dietary [13,14], metabolic [15] and pharmacological manipulations [16,17] according to previous reports.
Furthermore, ATP-binding cassette transporter A1 (ABCA1) is supposed to possess a role in cellular cholesterol efflux and HDL formation [18,19]. Some research reported that dietary supplement of Curcumin with moderated intake of alcohol increased cholesterol efflux mediated by ABCA1 [20,21]. However, according to a recent study, hepatic ABCA1 may not be the main contributing factor of HDL metabolism in a model of obesity-induced hyperinsulinemia using human apolipopretein B100 transgenic/brown adipose tissue deficient mice [22]. Thus, there is still controversy about the role of ABCA1 in regulating the HDL-cholesterol level.
In obese condition, abnormal lipid metabolism in the liver causes systemic dyslipidemia including skin. Previous studies have been reported the abnormal skin physiology and the delayed wound healing in obese subjects [23-25].
Our previous studies have reported that combined mulberry leaf and fruit extract (MLFE) rich in polyphenols have shown anti-obesity and anti-inflammatory effects including beneficial effect on dyslipidemia in the high fat diet induced obese mice [26,27].
Therefore, the aims of this study were to determine the expression of SR-B1 and ABCA1 liver and skin in high fat diet-induced obese mice and to investigate the effect of MLFE on cholesterol metabolism mediated by these markers.
The dried leaves (1.0 kg) and fruits (1.0 kg) were extracted with 70% ethanol. The mixture was filtered, evaporated in a rotary evaporator, and lyophilized. Using this procedure, the yield was 20% and 28% of the starting dry weight of mulberry leaves and fruits, respectively. The obtained ethanol extract of mulberry leaves (MLE) and fruits (MFE) were kept at -20℃ until it was used.
Male 4-week-old C57BL/6 mice were obtained from Orient Bio Inc (South Korea) and were housed at a constant temperature (22 ± 1℃) with a 12 h dark/light cycle and given access to tap water and food ad libitum. After a 1-week acclimation period, the animals were randomly divided into two groups, a control diet group (CON) and a high fat diet group (HF). Each group was treated with either the CON diet (D12450B, 10% kcal fat; Research Diets, New Brunswick, NJ, USA) or the HF diet (D12451, 45% kcal fat; Research Diets) for 9 weeks, respectively. All mice were used in accordance with animal protocols approved by Kyung Hee University Institutional Laboratory Animal Care and Use Committee (KHUASP(SE) -10-022).
After obesity was induced by the HF diet for 9 weeks, the animals were divided into three groups with six mice in each group and treated as follows: CON: Non-obese control mice with distilled water; HF: obese mice with distilled water; MLFE: obese mice with 1:1 ratio of mulberry leaf ethanol extract (MLE) and mulberry fruit ethanol extract (MFE) at a dose of 500mg/kg/day (250mg/kg/day, 250mg/kg/day, respectively). Treatments were administered by gavage 5 times weekly for 12 weeks. During the experiment period, body weights were recorded weekly. At the end of the experimental period, the animals were anesthetized by mixture of Zoletil 50® (Virbac, Carros, France) and Rompun® (Bayer Korea, Seoul, South Korea) solution (3:1 ratio, 1 ml/kg, i.p.) The blood was collected from the postcaval vein into heparin-coated tubes to analyze biochemical parameters. Plasma was prepared by centrifugation at 3000 rpm for 15 min, and frozen.
Plasma triglycerides (TG), total cholesterol (TC), and high-density lipoprotein (HDL) cholesterol levels were measured enzymatically using a commercial kit (Bio Clinical System Co., South Korea).
Liver and skin tissue were fixed in 10% buffered formaldehyde and embedded in paraffin. For histological observation, the sections (4 µm thickness) were deparaffinized in xylene and rehydrated in alcohol gradients and then stained with hematoxylin and eosin (H&E). For immunohistochemistry, the sections were dewaxed and rehydrated with the same method for histology. After dewaxing, the slides were incubated overnight at 4℃ with following antibodies: 4-HNE (Millipore Corporation, Billerica, MA, USA), SR-B1 (Novus Biologicals, Inc.; Littleton, CO) and ABCA1 (Abcam, Cambridge, MA). Then the slides were washed three times with phosphate-buffered saline (PBS), and endogenous peroxidase was blocked with 3% H2O2 in absolute methanol for 30 minutes at room temperature (RT). Next, the slides were incubated with EnVision+ System-HRP (DAKO, Glostrup, Denmark) for 45 minutes at RT. Finally, the reaction products were stained with diaminobenzidine (DAB), counterstained with Mayer's hematoxylin, and mounted with Eukitt mounting medium after drying. Images were acquired and analyzed with Axio Vision Release 4.6.3 software.
The body weight gains in HF diet-fed obese controls were significantly higher than those of lean controls. Mice treated with MLFE gained body weight less than those treated with only the HF diet. Plasma TC and TG levels were higher in the HF group than those in lean controls, but MLFE treatment lowered TC levels observed in the HF group. No differences in HDL-C levels were found among the groups (Table 1).
Histological analyses showed that livers in the HF group had macrovesicular and microvesicular steatosis, whereas the MLFE treatments showed clearly a lesser accumulation of fat in the liver tissues (Fig. 1 upper panel). On the other hand, no significant modifications were noticed in skin tissues (Fig. 1 lower panel).
It has been well demonstrated that an HF diet correlates with an increased systemic oxidative stress; therefore, we wanted to determine whether also in our system there was an increased oxidative stress status. To do so, we measured the formation of the alpha-beta unsaturated aldehyde 4-HNE, a reliable marker of the lipid peroxidation, which is induced by redox imbalance. As shown in Fig. 2A, a higher number of 4-HNE positive liver cells were observed in the HF group in comparison with the CON group (Fig. 2C). When the animals were treated with MLFE, the levels of liver 4-HNE was significantly reduced to the levels of the controls. 4-HNE was strongly produced almost all the hepatocytes in the HF group.
Both SR-B1 and ABCA1 are involved in cholesterol transport; therefore, we were interested in evaluating whether the HF diet and MLFE treatments were able to modulate their expressions. As shown in Fig. 3A, SR-B1 expression in both HF treated animals was very evident, showing a location of SR-B1 mainly seen in hepatocytes surrounding periportal spaces and hepatic venules (as appreciable in the detailed picture) (arrows). On the other hand, a significant reduction of SR-B1 levels was observed in MLFE group with levels similar to the controls (Fig. 3C).
As shown in Fig. 4, the expression of both SR-B1 and ABCA1 in cutaneous tissue had a different trend respect to the liver (Fig. 3). SR-B1 levels in the HF group were significantly increased (2 fold), and it was mainly present in the epidermis with less positivity in the dermis (Fig. 3C). MLFE treatment did not affect HF induced SR-B1 expression remaining at the same level of HF diet fed animals (Fig. 4A).
A similar trend was noticed for ABCA1 expression (Fig. 4B), with a strong induction by HF (3 fold) and also in this case MLFE treatment did not affect HF diet induced ABCA1 expression (Fig. 4C). In all groups, ABCA1 was mainly expressed in the epidermis, but it is possible to notice its presence in the basal dermis layer.
The present study demonstrated that MLFE supplementation is able to modulate proteins related to cholesterol transport, SRB-1 and ABCA-1 in liver and cutaneous tissue accompanied by reduction of oxidative stress demonstrated by 4-HNE in HF induced obese mice.
It has been well demonstrated that obesity and also a high fat diet are strong inducers of oxidative stress, and among the many biological targets of oxidative stress, lipids peroxidation is the most prominent [28,29]. Lipid peroxidation generates a number of byproducts and the unsaturated aldehydes such as 4-HNE. 4-HNE is the most toxic molecule among them and also a reliable biomarker of lipid peroxidation and oxidative stress. 4-HNE derives from the oxidation of ω-6 PUFAs, essentially arachidonic and linoleic acids, which are the most represented fatty acids in bio-membranes. Virtually, all of the biochemical effects of 4-HNE can be explained by its high reactivity towards the thiol and amino groups of amino acids. Primary reactants for 4-HNE are the amino acids cysteine, histidine and lysine, which - either free or protein-bound - undergo readily Michael addition reactions to the C = C double bond. In a previous study, we have demonstrated that 4-HNE is able to bind to the SH groups present on SR-B1, which is a mechanism involved in SR-B1 degradation [30]. In addition, we have also previously demonstrated that oxidative stress down-regulates SR-B1 expression, mainly via posttranslational modifications [31,32]. In the present work, although the increased levels of oxidative stress was demonstrated by 4-HNE in liver and skin tissue of the animals fed HF we detected increased levels of SR-B1 in the same tissues of the HF group. This apparently controversial data can be explained by the fact that SR-B1 has been shown to be modulated by cholesterol levels as well. Spady et al. have shown that SR-B1 expression and HDL cholesteryl ester transport were increased in animals fed polyunsaturated fatty acids [13]. On the other hand, other researchers have shown a reduction of hepatic SR-B1 in rats fed with a diet containing 2% cholesterol [33]. Thus, it suggests that the modulation of SR-B1 by cholesterol is not very simple. Moreover, it should be taken into consideration that the SR-B1 gene contains the consensus site for steroidogenic factor-1 (SF-1), which is very low in the liver. Although several other factors are involved in SR-B1 gene regulation, the exact mechanism is still under investigation. In our model, it is a possible scenario where, from one side, the presence of 4-HNE will induce the loss of SR-B1, and from the other side, other factors such as increased levels of cholesterol induce the expression of SR-B1. As a result, the levels of SR-B1 will be stronger inputs determined at both transcriptional and post-transductional levels.
The effect of MLFE treatment on SR-B1 liver levels in the HF fed animals can be conducted to its ability to decrease cholesterol levels so as to quench the modulation by cholesterol on SR-B1 expression. This effect was not appreciated in cutaneous tissue, and this could be a consequence of the different location of these organs. Most likely MLFE taken by gavage will hardly reach an appreciable level in cutaneous tissues, and so its effect on SR-B1 expression in the skin is less evident compared to the liver.
SR-B1 has also been shown to be expressed in several tissues other than the liver, including the lung, ovary, testis, brain, spleen, kidney [34] and, recently, even skin [35]. The role of SR-B1 in cutaneous tissue could be related to its ability to regulate cholesterol trafficking as suggested by Tsuruoka et al. [35], demonstrating that SR-B1 levels decreased during keratinocytes differentiation. Therefore, we can speculate that the HF would affect skin physiology modulating keratinocytes differentiation.
In our model, we noticed the presence of fatty liver in the HF fed animals, and this can be linked to increased lipid peroxidation, since peroxidation of membrane lipids is associated with steatosis [36]. The aldehyde product of lipid peroxidation induces activation of hepatic stellate cells [37], which are the main collagen-producing cells within the liver, leading to enhanced extracellular matrix protein deposition. In addition, peroxidation products may also contribute to inflammation by activating nuclear factor-kappa B (NF-κB), a transcription factor regulating the expression of several pro-inflammatory cytokines and adhesion molecules [38-40] and ROS production [30] such as superoxide anions capable of initiating lipid peroxidation and enhanced formation of ROS, leading to increased oxidative stress. In this respect, MLFE seems to have an effect on the reduction of lipid peroxidation, as demonstrated in our study, where we have found significantly reduced 4-HNE levels in liver tissues.
The current study showed that liver ABCA1 levels were increased in the HF group. ABC transporters are linked to cholesterol transport. Cholesterol homeostasis results from the balance between the de novo synthesis of cholesterol, the uptake of cholesterol from lipoproteins, and cholesterol efflux. The efflux of cholesterol from cells is mediated predominantly by ABCA1, which is a member of the ATP binding cassette transporter superfamily [41,42]. ABCA1 promotes the transport of cholesterol and phospholipids across cell membranes, where these lipids then complex with lipoproteins [42]. Moreover, ABCA1 has been shown to be susceptible to oxidative stress [43] with an increased expression. This is in agreement with the data presented herein, where we detected significant an increase in ABCA1 in the HF fed animals. The MLFE treatment was able to counteract the HF induced ABCA1 expression, and this could be also a consequence of the antioxidant properties of MLFE that parallel with the lower level of 4-HNE found in the liver of the MLFE-treated group. There were not significant effects of MLFE supplementation on levels of SR-B1 and ABCA1 in skin tissue. This could be still the consequence of the different tissue location, and it would be of interest to understand whether a topical application of MLFE would be beneficial for cutaneous treatments. Of interest is a recent paper from our group showing that CS exposure was able to upregulate ABCA1 expression in keratinocytes via the nuclear translocation of liver X receptor (LXR), a well-known orphan receptor via the modulation of NFκB, which is a regulator of LXR [44].
Our group has already demonstrated that the combined MLE and MFE treatments with the HF were responsible for prevention of body weight gain compared with the HF group in the previous studies and this is the follow-up study of the work in the previous studies [26,27]. This is the follow-up study of the work in the previous studies [26,27]. Our group has suggested that the combined MLFE supplementation has a beneficial effect on lipid metabolism, including cholesterol and fat accumulation in HF diet induced obese mice. Other studies have shown that mulberry leaves containing flavonoids reduced plasma TG, TC, and LDL cholesterol in hyperlipidemic rats as well [45,46]. Furthermore, mulberry fruit showed a beneficial effect on blood lipid profiles and lipid metabolism in hyperlipidemic rats [47]. Our data showed that the MLFE treatments significantly reduced plasma TG levels and decreased atherogenicity, as shown by a reduced AI compared with those in the HF group. These results indicate that the MLFE treatments might have a beneficial effect on TG and cholesterol metabolism in obese rats.
Increased liver TG depots cause further fat accumulation in hyperlipidemic mice [48]. Our histological examination showed that livers in the HF group developed macrovesicular and microvesicular steatosis, whereas MLFE treatment revealed decreased liver fat accumulation in the HF fed animals.
In conclusion, the present study demonstrated that an MLFE supplementation would modulate HF induced oxidative stress and regulate cholesterol transport proteins in liver and cutaneous tissue. These results suggest that MLFE might be a beneficial substance for therapies to prevent obesity related complications in liver and/or skin which are related to abnormal lipid metabolism.
Figures and Tables
Fig. 1
Effect of MLFE supplementation on liver (A) and skin (B) morphology in high fat diet-induced obese mice. (magnitude × 100) CON: the mice fed the control diet, HF: the mice fed the high fat diet, MLFE: the mice fed the HF group administered with 1:1 ratio of MLE and MFE at dose of 500 mg/kg/day.
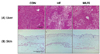
Fig. 2
Effect of MLFE supplementation on 4-HNE expression of liver (A) and skin (B) in high fat diet-induced obese mice. (magnitude × 100) (C) is a quantitative estimation for 4-HNE. CON: the mice fed the control diet, HF: the mice fed the high fat diet, MLFE: the mice fed the HF group administered with 1:1 ratio of MLE and MFE at dose of 500 mg/kg/day. *significant vs respective control. #significant within the same group. P < 0.05
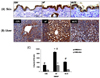
Fig. 3
Effect of MLFE supplementation on liver SR-B1 (A) and ABCA1 (B) expressions in high fat diet-induced obese mice. (magnitude × 100) (C) is a quantitative estimation for SR-B1 and ABCA1. CON: the mice fed the control diet, HF: the mice fed the high fat diet, MLFE: the mice fed the HF group administered with 1:1 ratio of MLE and MFE at dose of 500 mg/kg/day. *significant vs respective control. #significant within the same group. P < 0.05
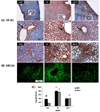
Fig. 4
Effect of MLFE supplementation on skin SR-B1 (A) and ABCA1 (B) expressions in high fat diet-induced obese mice. (magnitude × 100) (C) is a quantitative estimation for SR-B1 and ABCA1. CON: the mice fed the control diet, HF: the mice fed the high fat diet, MLFE: the mice fed the HF group administered with 1:1 ratio of MLE and MFE at dose of 500 mg/kg/day. *significant vs respective control. #significant within the same group. P < 0.05
Table 1
Effect of MLFE supplementation on body weight gain and plasma lipid profiles in high fat diet-induced obese mice

CON: the mice fed the control diet, HF: the mice fed the high fat diet, MLFE: the mice fed the HF group administered with 1:1 ratio of MLE and MFE at dose of 500 mg/kg/day. AI: atherogenic index = [(TC) - (HDL-C)] / (HDL-C). All values are means ± S.E.M. (n = 5-6) Mean values with unlike letters were significantly different (P < 0.05).
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2010-0006624).
References
1. Brundtland GH. From the World Health Organization. Reducing risks to health, promoting healthy life. JAMA. 2002; 288:1974.
2. Fernández-Sánchez A, Madrigal-Santillán E, Bautista M, Esquivel-Soto J, Morales-González A, Esquivel-Chirino C, Durante-Montiel I, Sánchez-Rivera G, Valadez-Vega C, Morales-González JA. Inflammation, oxidative stress, and obesity. Int J Mol Sci. 2011; 12:3117–3132.

4. Esposito K, Ciotola M, Schisano B, Misso L, Giannetti G, Ceriello A, Giugliano D. Oxidative stress in the metabolic syndrome. J Endocrinol Invest. 2006; 29:791–795.

5. Pitocco D, Zaccardi F, Di Stasio E, Romitelli F, Santini SA, Zuppi C, Ghirlanda G. Oxidative stress, nitric oxide, and diabetes. Rev Diabet Stud. 2010; 7:15–25.

6. Mukhtar H, Elmets CA. Photocarcinogenesis: mechanisms, models and human health implications. Photochem Photobiol. 1996; 63:356–357.

7. Shindo Y, Witt E, Packer L. Antioxidant defense mechanisms in murine epidermis and dermis and their responses to ultraviolet light. J Invest Dermatol. 1993; 100:260–265.

8. Shindo Y, Witt E, Han D, Epstein W, Packer L. Enzymic and non-enzymic antioxidants in epidermis and dermis of human skin. J Invest Dermatol. 1994; 102:122–124.

9. Mattson MP. Roles of the lipid peroxidation product 4-hydroxynonenal in obesity, the metabolic syndrome, and associated vascular and neurodegenerative disorders. Exp Gerontol. 2009; 44:625–633.

10. Vincent HK, Powers SK, Dirks AJ, Scarpace PJ. Mechanism for obesity-induced increase in myocardial lipid peroxidation. Int J Obes Relat Metab Disord. 2001; 25:378–388.

11. Fon Tacer K, Rozman D. Nonalcoholic Fatty liver disease: focus on lipoprotein and lipid deregulation. J Lipids. 2011; 2011:783976.

12. Trigatti BL, Krieger M, Rigotti A. Influence of the HDL receptor SR-BI on lipoprotein metabolism and atherosclerosis. Arterioscler Thromb Vasc Biol. 2003; 23:1732–1738.

13. Spady DK, Kearney DM, Hobbs HH. Polyunsaturated fatty acids up-regulate hepatic scavenger receptor B1 (SR-BI) expression and HDL cholesteryl ester uptake in the hamster. J Lipid Res. 1999; 40:1384–1394.

14. Hatahet W, Cole L, Kudchodkar BJ, Fungwe TV. Dietary fats differentially modulate the expression of lecithin:cholesterol acyltransferase, apoprotein-A1 and scavenger receptor b1 in rats. J Nutr. 2003; 133:689–694.

15. Roberts CK, Liang K, Barnard RJ, Kim CH, Vaziri ND. HMG-CoA reductase, cholesterol 7alpha-hydroxylase, LDL receptor, SR-B1, and ACAT in diet-induced syndrome X. Kidney Int. 2004; 66:1503–1511.

16. Grefhorst A, Oosterveer MH, Brufau G, Boesjes M, Kuipers F, Groen AK. Pharmacological LXR activation reduces presence of SR-B1 in liver membranes contributing to LXR-mediated induction of HDL-cholesterol. Atherosclerosis. 2012; 222:382–389.

17. Datar R, Kaesemeyer WH, Chandra S, Fulton DJ, Caldwell RW. Acute activation of eNOS by statins involves scavenger receptor-B1, G protein subunit Gi, phospholipase C and calcium influx. Br J Pharmacol. 2010; 160:1765–1772.

18. Attia N, Fournier N, Vedie B, Cambillau M, Beaune P, Ziegler O, Grynberg A, Paul JL, Guerci B. Impact of android overweight or obesity and insulin resistance on basal and postprandial SR-BI and ABCA1-mediated serum cholesterol efflux capacities. Atherosclerosis. 2010; 209:422–429.

19. Villarreal-Molina MT, Aguilar-Salinas CA, Rodríguez-Cruz M, Riaño D, Villalobos-Comparan M, Coral-Vazquez R, Menjivar M, Yescas-Gomez P, Königsoerg-Fainstein M, Romero-Hidalgo S, Tusie-Luna MT, Canizales-Quinteros S. Metabolic Study Group. The ATP-binding cassette transporter A1 R230C variant affects HDL cholesterol levels and BMI in the Mexican population: association with obesity and obesity-related comorbidities. Diabetes. 2007; 56:1881–1887.

20. Dong SZ, Zhao SP, Wu ZH, Yang J, Xie XZ, Yu BL, Nie S. Curcumin promotes cholesterol efflux from adipocytes related to PPARgamma-LXRalpha-ABCA1 passway. Mol Cell Biochem. 2011; 358:281–285.

21. Beulens JW, Sierksma A, van Tol A, Fournier N, van Gent T, Paul JL, Hendriks HF. Moderate alcohol consumption increases cholesterol efflux mediated by ABCA1. J Lipid Res. 2004; 45:1716–1723.

22. Ehlers SJ, Larson SM, Rasmussen HE, Park YK, Lee JY. High-density lipoprotein metabolism in human apolipoprotein B(100) transgenic/brown adipose tissue deficient mice: a model of obesity-induced hyperinsulinemia. Appl Physiol Nutr Metab. 2011; 36:313–322.

23. Yosipovitch G, DeVore A, Dawn A. Obesity and the skin: skin physiology and skin manifestations of obesity. J Am Acad Dermatol. 2007; 56:901–916.

24. Seitz O, Schürmann C, Hermes N, Müller E, Pfeilschifter J, Frank S, Goren I. Wound healing in mice with high-fat diet- or ob gene-induced diabetes-obesity syndromes: a comparative study. Exp Diabetes Res. 2010; 2010:476969.
25. Wilson JA, Clark JJ. Obesity: impediment to wound healing. Crit Care Nurs Q. 2003; 26:119–132.
26. Lim HH, Yang SJ, Kim Y, Lee M, Lim Y. Combined treatment of mulberry leaf and fruit extract ameliorates obesity-related inflammation and oxidative stress in high fat diet-induced obese mice. J Med Food. 2013; 16:673–680.

27. Lim HH, Lee SO, Kim SY, Yang SJ, Lim Y. Anti-inflammatory and antiobesity effects of mulberry leaf and fruit extract on high fat diet-induced obesity. Exp Biol Med (Maywood). 2013; 238:1160–1169.

28. Barrera G. Oxidative stress and lipid peroxidation products in cancer progression and therapy. ISRN Oncol. 2012; 2012:137289.

29. Bełtowski J, Wójcicka G, Górny D, Marciniak A. The effect of dietary-induced obesity on lipid peroxidation, antioxidant enzymes and total plasma antioxidant capacity. J Physiol Pharmacol. 2000; 51:883–896.
30. Sticozzi C, Belmonte G, Pecorelli A, Arezzini B, Gardi C, Maioli E, Miracco C, Toscano M, Forman HJ, Valacchi G. Cigarette smoke affects keratinocytes SRB1 expression and localization via H2O2 production and HNE protein adducts formation. PLoS One. 2012; 7:e33592.

31. Valacchi G, Davis PA, Khan EM, Lanir R, Maioli E, Pecorelli A, Cross CE, Goldkorn T. Cigarette smoke exposure causes changes in Scavenger Receptor B1 level and distribution in lung cells. Int J Biochem Cell Biol. 2011; 43:1065–1070.

32. Sticozzi C, Belmonte G, Pecorelli A, Cervellati F, Leoncini S, Signorini C, Ciccoli L, De Felice C, Hayek J, Valacchi G. Scavenger receptor B1 post-translational modifications in Rett syndrome. FEBS Lett. 2013; 587:2199–2204.

33. Fluiter K, Vietsch H, Biessen EA, Kostner GM, van Berkel TJ, Sattler W. Increased selective uptake in vivo and in vitro of oxidized cholesteryl esters from high-density lipoprotein by rat liver parenchymal cells. Biochem J. 1996; 319:471–476.

34. Mardones P, Strobel P, Miranda S, Leighton F, Quiñones V, Amigo L, Rozowski J, Krieger M, Rigotti A. Alpha-tocopherol metabolism is abnormal in scavenger receptor class B type I (SR-BI)-deficient mice. J Nutr. 2002; 132:443–449.

35. Tsuruoka H, Khovidhunkit W, Brown BE, Fluhr JW, Elias PM, Feingold KR. Scavenger receptor class B type I is expressed in cultured keratinocytes and epidermis. Regulation in response to changes in cholesterol homeostasis and barrier requirements. J Biol Chem. 2002; 277:2916–2922.

36. Morán-Ramos S, Avila-Nava A, Tovar AR, Pedraza-Chaverri J, López-Romero P, Torres N. Opuntia ficus indica (nopal) attenuates hepatic steatosis and oxidative stress in obese Zucker (fa/fa) rats. J Nutr. 2012; 142:1956–1963.

37. Parola M, Pinzani M, Casini A, Albano E, Poli G, Gentilini A, Gentilini P, Dianzani MU. Stimulation of lipid peroxidation or 4-hydroxynonenal treatment increases procollagen α1 (I) gene expression in human liver fat-storing cells. Biochem Biophys Res Commun. 1993; 194:1044–1050.

38. Sarada S, Himadri P, Mishra C, Geetali P, Ram MS, Ilavazhagan G. Role of oxidative stress and NFkB in hypoxia-induced pulmonary edema. Exp Biol Med (Maywood). 2008; 233:1088–1098.

39. Kalpana S, Dhananjay S, Anju B, Lilly G, Sai Ram M. Cobalt chloride attenuates hypobaric hypoxia induced vascular leakage in rat brain: molecular mechanisms of action of cobalt chloride. Toxicol Appl Pharmacol. 2008; 231:354–363.

40. Hang CH, Shi JX, Li JS, Wu W, Yin HX. Concomitant upregulation of nuclear factor-kB activity, proinflammatory cytokines and ICAM-1 in the injured brain after cortical contusion trauma in a rat model. Neurol India. 2005; 53:312–317.

41. Oram JF, Vaughan AM. ABCA1-mediated transport of cellular cholesterol and phospholipids to HDL apolipoproteins. Curr Opin Lipidol. 2000; 11:253–260.

42. Srivastava N. ATP binding cassette transporter A1--key roles in cellular lipid transport and atherosclerosis. Mol Cell Biochem. 2002; 237:155–164.
43. Valacchi G, Vasu VT, Yokohama W, Corbacho AM, Phung A, Lim Y, Aung HH, Cross CE, Davis PA. Lung vitamin E transport processes are affected by both age and environmental oxidants in mice. Toxicol Appl Pharmacol. 2007; 222:227–234.

44. Sticozzi C, Pecorelli A, Belmonte G, Valacchi G. Cigarette smoke affects ABCAl expression via liver X receptor nuclear translocation in human keratinocytes. Int J Mol Sci. 2010; 11:3375–3386.

45. Kim SY, Lee WC, Kim HB, Kim AJ, Kim SK. Antihyperlipidemic effects of methanol extracts from mulberry leaves in cholesterol-induced hyperlipidemia rats. J Korean Soc Food Sci Nutr. 1998; 27:1217–1222.
46. Park SH, Jang MJ, Hong JH, Rhee SJ, Choi KH, Park MR. Effects of mulberry leaf extract feeding on lipid status of rats fed high cholesterol diets. J Korean Soc Food Sci Nutr. 2007; 36:43–50.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download