Abstract
BACKGROUND
Groundwater is believed to possess many beneficial effects due to its natural source of various minerals. In this study, we examined the effects of natural Jeju groundwater S1 (Samdasoo™), S2 and S3 pumped up from different locations of Jeju Island, Korea, along with local tap water, on body weight gain, serum lipids and lipoproteins, and liver histopathology in high-fat diet-induced hyperlipidemic rats.
MATERIALS/METHODS
Rats were randomly and equally divided into 6 groups. Different water samples were supplied to the hyperlipidemic rats as their daily drinking water and the widely-used anti-hyperlipidemic drug simvastatin was used as a positive control. Body weight, serum lipids and lipoproteins were measured weekly. Liver weight, liver index and liver histopathology were examined after the execution of the rats.
RESULTS
After drinking Jeju groundwaters for two months, S2 but not S3 significantly reduced weight growth and serum triglycerides levels and increased high density lipoprotein-C (HDL-C) without affecting total cholesterol or LDL-C. S1 and particularly S2 significantly reduced the severity of liver hypertrophy and steatosis. All Groundwaters had much higher contents of vanadium (S3>S2>S1>>tap water) whereas S1 and S2 but not S3 markedly blocked autoxidation of ferrous ions.
Groundwater is rich in minerals and dissolved substances through chronic interactions with geologic formations. The unique geochemistry characteristic both in common elements (Na+, K+, Mg2+, Ca2+, HCO3-, Cl-, SO42-, NO3- and SiO32-) and trace elements give groundwaters special taste as well as nutritional or therapeutic values [1], especially in metabolic diseases like diabetes and obesity. Common elements invariably make up over 99% of the solute contents in nature waters [1] and determine the main property such as hardness and pH of the water. Hard waters, which are sufficient in magnesium and calcium, were reported to reduce liver cholesterol concentrations [2] and serum total cholesterol [3]. Such benefits may be contributed from inhibition of fat absorption [4,5,6], cholesterol absorption [5,6,7] and bile salts absorption [6,8].
Except for common elements, more therapeutic values of groundwater are probably due to trace elements, in which vanadium has been identified to be insulin-mimic, including increasing glucose transport and oxidation in adipocytes, stimulating glycogen synthesis and inhibiting gluconeogenesis in hepatocytes [9]. Indeed, a recent study elucidated the effects of natural vanadium-containing Jeju groundwater on glucose uptake in L6 myotubes and adipogensesis in 3T3L1 cells. Cultured media which contains Jeju groundwater was found to stimulate glucose uptake in a vanadium-dependent manner [10]. Besides its hypoglycemic effects, vanadium also exhibited effects on lipid metabolism. Vanadium inhibited cholesterol synthesis [11,12]. Animal experiments also showed that oral administration of vanadium compounds reduced serum lipid in diabetic rats but not in normal rats [13], although whether there is an anti-lipid effect of vanadium in a small amount contained in natural groundwater is not clear.
Jeju Island is located at the southern part of the Korean Peninsula, formed by several volcanic explosions, and has a huge pool of groundwater. The present study aimed to investigate the possible beneficial effects of natural Jeju groundwater S1 (Samdasoo™), S2 and S3 which were pumped up from different locations under Jeju Island on lipid metabolism in high-fat diet-induced hyperlipidemic rats. We also measured the vanadium contents in S1, S2 and S3 and their ferrous ion autoxidation activity in an effort to illustrate the role of vanadium in Jeju natural Groundwaters on lipid metabolism. Our results showed that S1 and particularly S2, but not S3, exhibited marked protective effects against hypolipidemia and fatty liver in hyperlipidemic rats. S1, S2, and S3 had the same high contents of vanadium, but only S1 and S2 showed a blockade of ferrous ion autoxidation. The results suggest that beneficial effects of Jeju groundwaters on lipid metabolism in vivo may not be contributed from high contents of vanadium, although the underlying mechanism is not known.
Natural volcanic Groundwater S1 (Samdasoo™), S2 and S3 were pumped up from different locations under Jeju Island and provided by Jeju Special Self-Governing Province Development Corp. (Jeju, Korea), while local tap water was boiled as a standard before use. Jeju groundwaters were kept in sealed bottles stored at room temperature for approximately 4 months. Simvastatin was a gift from Shanghai Modernization Pharmaceuticals (Shanghai, China).
Male 6-8 week Sprague-Dawley rats (weighing 240-270 g), purchased from Shanghai Laboratory Animal Center (Shanghai, China), were kept in an environment of 20℃, 40-60% humidity and 12 h light/dark cycle (lights on 7:00 AM). Rats were housed for 3 days with free access to normal rat chow diet and tap water before experiment. Two rat chow diets, i.e., normal diet and high-fat diet were purchased from Shanghai Laboratory Animal Center. The components of normal and high-fat diet were summarized in Table 1. All rats were given free access to normal chow diet and tap water for 3 days and then randomly divided into 6 groups (n = 8 in each group). The rats got free access to the following for 7 weeks: 1) normal chow diet and tap water; 2) high-fat diet and tap water; 3) high-fat diet and daily gavage of simvastatin (10 mg/kg/day) for 3 weeks starting from the fourth week after starting the experiment; 4) high-fat diet and natural Jeju groundwater S1; 5) high-fat diet and Groundwater S2; 6) high-fat diet and Groundwater S3. Body weight and blood lipids were measured weekly. The study was approved by the Laboratory Animal Use Committee of Shanghai Jiao Tong University School of Pharmacy (SJTU-PH-2011-17).
Major cations (K+, Ca2+, Na+, Mg2+, Va and SiO2), anions (HCO3-) and basic hydrogeochemical characteristics (alkalinity and total hardness) were analyzed using an inductively coupled plasma mass spectrometry (ICP-MS), silicon-molybdenum colorimetric method and phenolphthalein-methyl orange titration by ALS Analytical Testing Co. (Shanghai, China), with validated testing procedures according to the respective Chinese National Standards.
Blood samples were centrifuged at 4,000 rpm to obtain serum. Serum lipids were measured according to the enzymatic colorimetric method [15] using commercial reagents from Beijing BHKT Clinical Reagent Co. (Beijing, China). Accurately packed enzyme powder was dissolved in 10 ml buffer to make a working solution. The serum samples were reacted with the working solution at a ratio of 1:100 in 37℃ for 5 minutes. Results were read at 490 nm by a Microplate Reader (Multiskan MK3, Thermo Labsystems, Vantaa, Finland) and calculated by comparison with standard curves of lipids. High density lipoprotein-C (HDL-C) and low density lipoprotein-C (LDL-C) were measured according to the polyanion polymer/detergent method [16]. The reagents, bought from Shanghai Rongsheng Biotech Co. (Shanghai, China), were composed of the poly anion solution and the enzymatic solution. Serum samples were first reacted with the poly anion solution for 5 minutes at 37℃. Blank values were read at 490 nm by a microplate reader. The enzymatic solution was then added and other 5-min incubation was performed. The absorbance in 550 nm was determined to compare with the standard curve of lipoproteins.
At the end of the experiment, rats were anaesthetized and executed. The liver was quickly removed and weighed immediately using a 1-g scale balance. Their proportion in body weight was calculated as hepatosomatic index which equal to liver weight/body weight × 100. A patch of the liver was then collected and put into 10% formaldehyde solution. Fixed tissues were embedded in paraffin, cut into sections and placed on microscope slides. Slides were stained with hematoxylin and eosin. For histomorphological examination, images of different parts of each slide were taken under a 10 × 10 microscope (Olympus IX51, Tyoko, Japan). Fat vacuoles were blindly counted by using the Image-Pro Plus Software (Version 6.0.0.260, Media Cybernetics Inc, Bethesda, MD, USA) and divided by the area of the section.
For the ferrous ion autoxidation assay, 20 mg/ml ferrous ammonium sulfate solution was prepared by 1 ml sample water and was diluted 100 times. After 10 minutes, an equivalent volume of 1% potassium ferricyanide was added and the absorbance was read under the wavelength of 630 nm. The remaining ferrous ion concretion was calculated by the standard curve and the extent of ferrous ion autoxidation was evaluated: autoxidation rate (%) = (theoretic ferrous ion concretion - remained ferrous ion concretion) / theoretic ferrous concretion × 100.
For the radical scavenging activity assay, the effect of cwaters on the 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical was measured according to the method [14] with a minor modification. In principle, the purple color of DPPH becomes yellow if there is an antioxidant activity of tested solution. The 900 µl water sample was mixed with 300 µl DPPH-alcohol solution (1 mM) and votexed. The mixture was incubated at 37℃ for 30 minutes and read under 490 nm. Pure alcohol was used as a blank control. The scavenging activity was calculated using the following equation: radical scavenging activity (%) = [(control - sample)/control] × 100.
Results are presented as mean ± S.E.M and were tested by one-way or two-way repeated measures analysis of variance (ANOVA) followed by post-hoc Tukey's test by using Prism (version 5.01; Graphpad Software Inc., San Diego, CA, USA). P-value less than 0.05 were taken to indicate a statistically significant difference.
The mineral compositions and hydrochemical characteristics from Jeju groundwaters and tap water are shown in Table 2. As for hydrochemical characteristics, tap water could be classified as hard water according to its high concentration of Ca2+ and Mg2+ as well as its hardness value of over 150 mg/L. It is also considered as alkaline water with high alkalinity and high concentration of HCO3-. In contrast, S1, S2 and S3 had much lower concentrations of Ca2+ and Mg2+, resulting in lower hardness values and were considered as soft water. S3 had a relatively high alkalinity due to its bicarbonate contents while S1 and S2 had lower alkalinity. The identity of Jeju groundwater S1, S2 and S3 laid in their high vanadium contents of 6, 18 and 25 µg/L in contrast to the tap water value of 1 µg/L. S1, S2, and S3 also had dissolved SiO2 contents (38.0, 34.4 and 26.4 mg/L) that were significantly higher than tap water (5.4 mg/L).
As shown in Fig. 1A, rats fed with normal chow diet and tap water exhibited a normal serum triglycerides value of 1.9 mmol/L at the beginning of the experiment, with a progressive increase trend following breeding period. In rats drinking tap water, high-fat diet remarkably increased serum triglycerides levels (P < 0.05). Daily gavage of simvastatin (10 mg/kg/day) for three weeks (started on the fourth week) reduced high-fat diet-increased high serum triglycerides by 23.2% (P < 0.05) compared to the high-fat chow and tap water group, and reached the same level as in normal chow diet fed rats. Jeju groundwaters did not alter serum triglycerides levels during the drinking period of 4 weeks. However, Jeju groundwater S1 and particularly S2 (but not S3) produced mild decreases in serum triglycerides compared to the high-fat chow and tap water group; the drop rates for S1 and S2 were 8.3% (P > 0.05) and 16.6% (P < 0.05), respectively (Fig. 1B).
For serum levels of total cholesterol, rats fed with normal chow diet and tap water exhibited a normal serum total cholesterol value of 3.9 mmol/L at the beginning of the experiment, with a progressive increase trend following breeding period. In rats drinking tap water, high-fat diet remarkably increased serum total cholesterol levels (P < 0.05). Daily gavage of simvastatin (10 mg/kg/day) for three weeks (started on the forth week) reduced high-fat diet-increased high serum total cholesterol by 35.9% (P < 0.05) compared to the high-fat diet and tap water group, and reached the same level as in normal chow diet fed rats (Fig 2A). Jeju groundwaters S1, S2 and S3 slightly but insignificantly dropped serum total cholesterol levels in the seventh week by 7.1%, 20.3% and 9.9%, respectively (Fig. 2B).
High-fat diet lowered serum HDL-C significantly by 30.7% compared with normal chow fed rats (P < 0.05). Simultaneously, daily administration of simvastatin (10 mg/kg/day) raised serum HDL-C by 48.4% and reached the same level as in normal rats. Jeju groundwaters S1 and S2 (but not S3) also reversed the decreasing trend of serum HDL-C. HDL-C levels in these groups were increased significantly (P < 0.05) by 45.4% and 65.2% compared to the high-fat diet fed group (Fig 3A). As for serum LDL-C, high-fat diet group showed a markedly higher value (1.57 mmol/L) than the normal group (P < 0.05), but neither simvastatin nor any water samples showed significant inhibitory effects (Fig. 3B).
Rats fed with normal chow diet and tap water kept growing over the course of observation period. In rats drinking tap water, high-fat diet significantly increased body weight by 15.6% in the seventh week (P < 0.05), which was completely blocked by daily administrations of simvastatin (10 mg/kg/day) (P < 0.05) (Fig. 4A). Among three Jeju groundwaters, S1 and particularly S2 reduced high-fat-increased body weight by 7.7% (P > 0.05) and 17.7% (P < 0.05), respectively; S2 completely restored the body weight to the same level of the normal control (Fig. 4B).
Liver hypertrophy is common in the formation of fatty liver. At the end of the experiment, all rats were executed and livers were removed and weighed. As shown in Fig. 5A, the liver from the normal chow diet group exhibited blood like red color with weight of 17.9 g. In rats drinking tap water and high-fat diet, the liver was white in color and exhibited remarkable hypertrophy by increasing liver weight by 75.1% in the seventh week (P < 0.05). Daily injections of simvastatin (10 mg/kg/day) significantly reduced high-fat-increased liver weight by 35.4% to the level of the normal chow diet control (P < 0.05). Interestingly, Jeju groundwater S1 partially and S2 completely restored high-fat diet-increased liver hypertrophy to the same level of the normal chow diet control group (P < 0.05), with reduction of 20.0% and 31.8%, respectively, while S3 had no beneficial effect. In order to normalize liver weight with body weight, the Hepatosomatic index was employed. In rats drinking tap water high-fat diet increased the Hepatosomatic index by 50.0% (P < 0.05). Simvastatin (14.2%, P < 0.05), Jeju groundwater S1 (11.6%, P > 0.05) and S2 (16.7%, P < 0.05) (but not S3) moderately decreased the Hepatosomatic index (Fig. 5B).
In the liver histology examination, rats on the high-fat diet and tap water developed moderate hepatic steatosis compared to the rats on normal diet chow and tap water. The hepatocytes were occupied by high accumulation of fat in cytoplasm and showed a ballooning character. In contrast, few steatosis was observed in rats treated with simvastatin, S1 or S2. However, S3 treated rats showed comparable steastosis to the high-fat diet and tap water group. Representative photomicrographs from each group were shown in Fig. 6A. To confirm the initial observation, the hepatocytes with fat accumulation were blindly counted. As shown in Fig. 6B, few fatty vacuoles were observed in rats treated normal diet chow, while high-fat diet caused serious steatosis (P < 0.05). Simvastatin (60.7%), Jeju groundwater S1 (61.0%) and S2 (64.3%) (but not S3) markedly decreased fatty vacuoles (P < 0.05).
We were communicated that Jeju groundwaters blocked nail corrosion (Jeju Special-Governing Province Development Corp., personal communication). We also observed that white precipitation but not red rust appeared during the first a few days after immersing a nail with S1 and S2 but not S3. Stable for weeks, then finally turned to red, the white precipitation was totally dissolved in hydrochloric acid and the solution showed a shallow green color, an indicative of ferrous chloride. The observation indicated that S1 and S2 but not S3 might delay the oxidation of Fe(OH)2 to Fe2O3. To confirm this hypothesis, we investigated the effects of Jeju groundwaters on autoxidation of ammonium ferrous sulfate where ferrous ions are unstable and oxidized in oxygen-rich environments. Soon the ferrous ammonium sulfate solutions prepared by tap water and S3 became yellow. Further identification by potassium ferricyanide confirmed that over 70% ferrous ions had been oxidized in tab water and S3 with autoxidation rate of approximately 80%. In contrast, S1 and S2 maintained a shallow color and the extent of ferrous ion oxidation was markedly lower (P < 0.05) (Fig. 7A).
The desalinated Jeju underground seawater was reported to have a free radical scavenging activity [16]. To test whether Jeju groundwaters had similar effect, the in vitro DPPH assay was used. The treatments of all Jeju groundwaters (S1, S2 and S3) had significant higher absorbance compared to tap water, indicating their lower (instead of higher) radical scavenging activity (P < 0.05) (Fig 7B).
The present study discovered that drinking Jeju groundwater S1 and particularly S2 for 7 weeks produced marked hypolipidemic effects in high-fat diet-induced hyperlipidemic rats. The consistent results included the following: 1) S2 (and S1 slightly and insignificantly) but not S3 significantly reduced serum triglycerides and increased serum HDL-C while they did not significantly reduce serum total cholesterol or LDL levels;2) S2 and S1 but not S3 significantly reduced liver weight and the Hepatosomatic index; 3) S2 and S1 but not S3 significantly improved hepatic steatosis and reduced liver fatty vacuoles. The location-dependent hypolipidemic effects of the mineral waters collected under Hanna Mountain in Jeju Island is important particularly noting that they were comparable to that of simvastatin, a well-known lipid-lowering drug used clinically [17,18]. Our results are in agreement with a recent finding that drinking of the desalinated Jeju underground seawater for 10 weeks reduced hepatic triglyceride levels and steatosis without changing body weight and plasma lipid levels in high-fat diet fed mice, accompanied by a decrease in fatty acid synthase activity and an increase in carnitine palmitoyltransferase activity, two major enzymes in the synthesis and transport of fatty acid [16].
The underlying mechanisms and effective ingredients by which natural Jeju groundwaters produce nutritional/therapeutic hypolipidemic effects are completely unknown. We analyzed several dissolved elements and found that S1, S2 and S3 were unique in their high contents of vanadium in the range of 6, 16 and 25 µg/L, in contrast to local tap water (1 µg/L). The results are the same as a recent publication in which the vanadium contents were 8 µg/L (S1), 24 µg/L (S2) and 26 µg/L (S3), respectively [10]. Vanadium has been considered to have potential anti-hyperlipidemic effect. 2.5 mmol/L VOSO4·2H2O depressed the biosynthesis of cholesterol from labeled acetate in hepatic tissue of rats and the inhibition by vanadium occurred between the six and five carbon intermediates [11,19]. Vanadyl sulfate added in drinking water in the concentration of 0.2 mg/ml obviously lowered serum lipid value in streptozotocin-induced hyperglycemic rats during a 29 days treatment [13]. However, our data showed that Jeju groundwater S3 was not effective in reducing serum lipid level or liver hypertrophy although it had a high concentration of vanadium, even higher than that of S1 (+3 folds) and S2 (+72%), whereas S1 particularly S2 were anti-hyperlipidemic. Taken together these results may exclude the possibility that high concentration of vanadium is responsible for the anti-hyperlipidemic effect of natural Jeju groundwaters. The results also raise a question whether vanadium has a nutritional effect on lipid metabolism, as natural water, regardless of mineral or ground waters or tap water, hardly reaches the high level of vanadium as vanadium-added waters in the above biological studies.
Alternatively, we hypothesize that the blockade of ferrous ion autoxidation may contribute to reducing the oxidative damage and cellular toxicity in high-fat diet-induced metabolic syndrome. Iron, by far the most abundant transition metal in the body, can catalyze the formation of highly toxic hydroxyl radical through the Fenton reaction: Fe2+ +H2O2 → Fe3+ + -OH + ·OH, Fe3+ +O2· → Fe2+ +O2 [20]. The generated free radicals are known to induce oxidation of various biomolecules including lipids, lipoproteins, nucleic acids, and proteins [21]. Research on familial hypertriglyceridemic patients showed a clear relationship between high serum triglyceride and iron overlord [22]. A relative high serum triglycerides and cholesterol were observed in rats with high daily iron intake [23]. In metabolic liver diseases, hepatic iron overlord was seen in nonalcoholic fatty liver disease patients in several regions [24,25,26]. Iron overlord was also related to development of insulin resistance [25] and several kinds of metabolic syndrome indexes like body mass index, while triglycerides are significantly associated with ferritin values [27]. Although Jeju groundwaters do not have a free radical scavenging or antioxidative ability, S1 and S2 (but not S3) may be able to reduce free radicals during oxidation of ferrous ions, and are beneficial to lipid metabolism after multiple-daily intake. As S3 was ineffective in reducing ferrous ion autoxidation, vanadium should not be involved in S1 or S2-induced blockade of autoxidation of ferrous ions. Further work is needed to confirm the hypothesis in animal studies.
Figures and Tables
 | Fig. 1
Effects of simvastatin (10 mg/kg/day, A), Jeju groundwater S1 (Samdasoo™), S2 and S3 (B) on serum triglycerides levels in high-fat diet-induced hyperlipidemic rats. One group of rats with normal diet and tap water were also employed as the normal diet control. Data are presented as mean + S.E.M. (n = 8 in each group). a and b denote statistically significant difference (P < 0.05) compared with the normal chow diet + tap water group and high-fat diet + tap water group, respectively. |
 | Fig. 2
Effects of simvastatin (10 mg/kg/day, A), Jeju groundwater S1 (Samdasoo™), S2 and S3 (B) on serum total cholesterol levels in high-fat diet-induced hyperlipidemic rats. One group of rats with normal diet and tap water were also employed as the normal diet control. Data are presented as mean + S.E.M. (n = 8 in each group). a and b denote statistically significant difference (P < 0.05) compared with the normal chow diet + tap water group and high-fat diet + tap water group, respectively. |
 | Fig. 3
Effects of simvastatin (10 mg/kg/day), Jeju groundwater S1 (Samdasoo™), S2 and S3 on serum high density lipoprotein (HDL-C) levels (A) and low density lipoprotein (LDL-C) levels (B) in high-fat diet-induced hyperlipidemic rats. One group of rats with normal diet and tap water were employed as the normal diet control. Data are presented as mean + S.E.M. (n = 8 in each group). a and b denote statistically significant difference (P < 0.05) compared with the normal chow diet + tap water group and high-fat diet + tap water group, respectively. |
 | Fig. 4
Effects of simvastatin (10 mg/kg/day, A), Jeju groundwater S1 (Samdasoo™), S2 and S3 (B) on body weight in high-fat diet-induced hyperlipidemic rats. One group of rats with normal diet and tap water were also employed as the normal diet control. Data are presented as mean + S.E.M. (n = 8 in each group). a and b denote statistically significant difference (P < 0.05) compared with the normal chow diet + tap water group and high-fat diet + tap water group, respectively. |
 | Fig. 5
Effects of simvastatin (10 mg/kg/day), Jeju groundwater S1 (Samdasoo™), S2 and S3 on liver weight (A) and Hepatosomatic index (B) in high-fat diet-induced hyperlipidemic rats. One group of rats with normal diet and tap water were also employed as the normal diet control. Hepatosomatic index was calculated as liver weight/body weight × 100. Data are presented as mean + S.E.M. (n = 8 in each group). a and b denote statistically significant difference (P < 0.05) compared with the normal chow diet + tap water group and high-fat diet + tap water group, respectively. |
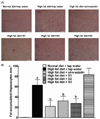 | Fig. 6
Effects of simvastatin (10 mg/kg/day), Jeju groundwater S1 (Samdasoo™), S2 and S3 on liver fat accumulation in high-fat diet-induced hyperlipidemic rats. One group of rats with normal diet and tap water were also employed as the normal diet control. (A) Representative photomicrographs from each group of rats. Slides were stained with hematoxylin and eosin and photos were taken in 1:400. (B) Liver fatty vacuoles from each group. Fat vacuoles were blindly counted with the assistance of a computer program. Data are presented as mean + S.E.M. (n = 8 in each group). a and b denote statistically significant difference (P < 0.05) compared with the normal chow diet + tap water group and high-fat diet + tap water group, respectively. |
 | Fig. 7
Effects of Jeju groundwater S1 (Samdasoo™), S2 and S3 on the autoxidation of ferrous ammonium sulfate (A) and radical (2,2-diphenyl-1-picrylhydrazyl, DPPH) scavenging activity (B). Data are presented as mean + S.E.M. (n = 6 in each group). a denotes statistically significant difference (P < 0.05) compared with the tap water group. |
References
1. Edmunds WM, Smedley PL. Groundwater geochemistry and health: an overview. Geol Soc Lond Spec Publ. 1996; 113:91–105.

2. Borgman RF, Lightsey SF. Effects of synthesized hard water and of cadmium in the drinking water upon lipid metabolism and cholelithiasis in rabbits. Am J Vet Res. 1982; 43:1432–1435.
3. Porter LP, Borgman RF, Lightsey SF. Effects of water hardness upon lipid and mineral metabolism in rabbits. Nutr Res. 1988; 8:31–45.

4. Denke MA, Fox MM, Schulte MC. Short-term dietary calcium fortification increases fecal saturated fat content and reduces serum lipids in men. J Nutr. 1993; 123:1047–1053.
5. Diersen-Schade DA, Richard MJ, Jacobson NL. Effects of dietary calcium and fat on cholesterol in tissues and feces of young goats. J Nutr. 1984; 114:2292–2300.

6. Yacowitz H, Fleischman AI, Amsden RT, Bierenbaum ML. Effects of dietary calcium upon lipid metabolism in rats fed saturated or unsaturated fat. J Nutr. 1967; 92:389–392.

7. Bhattacharyya AK, Thera C, Anderson JT, Grande F, Keys A. Dietary calcium and fat. Effect on serum lipids and fecal excretion of cholesterol and its degradation products in man. Am J Clin Nutr. 1969; 22:1161–1174.
8. Shahkhalili Y, Murset C, Meirim I, Duruz E, Guinchard S, Cavadini C, Acheson K. Calcium supplementation of chocolate: effect on cocoa butter digestibility and blood lipids in humans. Am J Clin Nutr. 2001; 73:246–252.

9. Tolman EL, Barris E, Burns M, Pansini A, Partridge R. Effects of vanadium on glucose metabolism in vitro. Life Sci. 1979; 25:1159–1164.
10. Hwang SL, Chang HW. Natural vanadium-containing Jeju ground water stimulates glucose uptake through the activation of AMP-activated protein kinase in L6 myotubes. Mol Cell Biochem. 2012; 360:401–409.

11. Curran GL. Effect of certain transition group elements on hepatic synthesis of cholesterol in the rat. J Biol Chem. 1954; 210:765–770.

12. Curran GL, Costello RL. Reduction of excess cholesterol in the rabbit aorta by inhibition of endogenous cholesterol synthesis. J Exp Med. 1956; 103:49–56.

13. Pepato MT, Magnani MR, Kettelhut IC, Brunetti IL. Effect of oral vanadyl sulfate treatment on serum enzymes and lipids of streptozotocin-diabetic young rats. Mol Cell Biochem. 1999; 198:157–161.
14. Noh JR, Gang GT, Kim YH, Yang KJ, Lee CH, Na OS, Kim GJ, Oh WK, Lee YD. Desalinated underground seawater of Jeju Island (Korea) improves lipid metabolism in mice fed diets containing high fat and increases antioxidant potential in t-BHP treated HepG2 cells. Nutr Res Pract. 2010; 4:3–10.

15. Trinder P. Determination of glucose in blood using glucose oxidase with an alternative oxygen acceptor. Ann Clin Biochem. 1969; 6:24–27.

16. Bachorik PS, Ross JW. National Cholesterol Education Program recommendations for measurement of low-density lipoprotein cholesterol: executive summary. The National Cholesterol Education Program Working Group on Lipoprotein Measurement. Clin Chem. 1995; 41:1414–1420.

17. Collins R, Armitage J, Parish S, Sleight P, Peto R. Heart Protection Study Collaborative Group. Effects of cholesterol-lowering with simvastatin on stroke and other major vascular events in 20536 people with cerebrovascular disease or other high-risk conditions. Lancet. 2004; 363:757–767.

18. Pedersen TR, Kjekshus J, Berg K, Haghfelt T, Faergeman O, Faergeman G, Pyörälä K, Miettinen T, Wilhelmsen L, Olsson AG, Wedel H. Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). 1994. Atheroscler Suppl. 2004; 5:81–87.

19. Azarnoff DL, Curran GL. Site of vanadium inhibition of cholesterol biosynthesis. J Am Chem Soc. 1957; 79:2968–2969.

20. Kruszewski M. The role of labile iron pool in cardiovascular diseases. Acta Biochim Pol. 2004; 51:471–480.

21. Pietrangelo A. Iron, oxidative stress and liver fibrogenesis. J Hepatol. 1998; 28:Suppl 1. 8–13.

22. Mateo-Gallego R, Calmarza P, Jarauta E, Burillo E, Cenarro A, Civeira F. Serum ferritin is a major determinant of lipid phenotype in familial combined hyperlipidemia and familial hypertriglyceridemia. Metabolism. 2010; 59:154–158.

23. Bristow-Craig HE, Strain JJ, Welch RW. Iron status, blood lipids and endogenous antioxidants in response to dietary iron levels in male and female rats. Int J Vitam Nutr Res. 1994; 64:324–329.
24. George DK, Goldwurm S, MacDonald GA, Cowley LL, Walker NI, Ward PJ, Jazwinska EC, Powell LW. Increased hepatic iron concentration in nonalcoholic steatohepatitis is associated with increased fibrosis. Gastroenterology. 1998; 114:311–318.

25. Hsiao TJ, Chen JC, Wang JD. Insulin resistance and ferritin as major determinants of nonalcoholic fatty liver disease in apparently healthy obese patients. Int J Obes Relat Metab Disord. 2004; 28:167–172.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download