Abstract
BACKGROUD/OBEJECTIVES
MATERIALS/METHODS
RESULTS
Figures and Tables
 | Fig. 1
Effects of peanut sprout extracts on PPARγ expression in adipose tissues of rats. Frozen adipose tissue was homogenized in lysis buffer. Protein concentration was determined by using a Bio-Rad method. Equal amounts of proteins (30 µg) were resolved by SDS-PAGE, transferred to the membranes and probed with PPARγ. Above photographs of chemiliuminiscent detection of the western blots, are shown and under the graph of quantitative analysis which were representatives of three independent experiments. Each bar represents the mean ± SE (n = 10). Comparison among different concentrations of peanut sprout extracts that yielded significant differences (P < 0.05) are indicated by the different letters above each bar. |
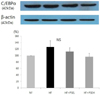 | Fig. 2
Effect of peanut sprout extracts on C/EBPα expression in adipose tissue of rats. Frozen adipose tissue was homogenized in lysis buffer. Protein concentration was determined by using a Bio-Rad method. Equal amounts of proteins (30 µg) were resolved by SDS-PAGE, transferred to the membranes and probed with C/EBPα. Above photographs of chemiliuminiscent detection of the western blots, are shown and under the graph of quantitative analysis which were representatives of three independent experiments. Each bar represents the mean ± SE |
 | Fig. 3
Effect of peanut sprout extracts on adiponectin expression in adipose tissue of rats. Frozen adipose tissue was homogenized in lysis buffer. Protein concentration was determined by using a Bio-Rad method. Equal amounts of proteins (30 µg) were resolved by SDS-PAGE, transferred to the membranes and probed with adiponectin. Above photographs of chemiliuminiscent detection of the western blots, are shown and under the graph of quantitative analysis which were representatives of three independent experiments. Each bar represents the mean ± SE Comparison among different concentrations of peanut sprout extracts that yielded significant differences (P < 0.05) are indicated by the different letters above each bar. |
 | Fig. 4
Effect of peanut sprout extracts on leptin protein expression in adipose tissue of rats. Frozen adipose tissue was homogenized in lysis buffer. Protein concentration was determined by using a Bio-Rad method. Equal amounts of proteins (30 µg) were resolved by SDS-PAGE, transferred to the membranes and probed with leptin. Above photographs of chemiliuminiscent detection of the western blots, are shown and under the graph of quantitative analysis which were representatives of three independent experiments. Each bar represents the mean ± SE |
Table 1

1) NF: normal-fat diets (7% fat diet), HF: high-fat diets (20% fat diet), HF + PSEL: high fat diets with low peanut sprout extract diet (20% fat and 15 mg/kg peanut sprout extract (0.025% resveratrol), and HF + PSEH: high fat with high peanut sprout extract diets (20% fat and 30 mg/kg peanut sprout extract (0.05% resveratrol).
2) Mineral mixture (per kg): Calcium carbonate anhydrous, 357 g; Potassium phosphate monobasic, 196 g; Potassium citrate tripotassium monohydrate, 70.78 g; Potassium sulfate Sodium chloride, 74 g: Magnesium oxide, 24 g; Ferric citrate, 6.06 g; Zinc carbonate, 1.65 g; Sodium meta-silicate, 1.45 g; Manganous carbonate, 0.63 g; Cupric carbonate, 0.30 g; Chromium potassium sulfate, 0.275 g; Boric acid, 81.5 mg; Sodium fluoride, 63.5 mg; Nickel carbonate, 31.8 mg; Lithium chloride, 17.4 mg; Sodium selenate anhydrous, 10.25 mg; Potassium iodate, 10.0 mg; Ammonium paramolybdate, 6.66 mg; Powdered sucrose, 221.026 g
3) Vitamin mixture (per kg) : Nicotinic acid, 3.0 g; Ca Pantothenate, 1.6 g; Pyridoxine HCl 0.7 g; Thiamin HCl, 0.6 g; Riboflavin 0.6 g; Folic acid, 0.2 g; Biotin, 0.02 g; Vitamin B12, 2.5 g; Vitamin E, 15.0 g; Vitamin A, 0.8 g; Vitamin D3, 0.25 g; Vitamin K-1, 0.075 g; Powdered sucrose, 974.655 g
* Peanut sprout water extracts: 38.17 mg/mL resveratrol




 PDF
PDF ePub
ePub Citation
Citation Print
Print







 XML Download
XML Download