Abstract
BACKGROUND/OBJECTIVES
In this study, the apoptogenic activity and mechanisms of cell death induced by hexane extract of aged black garlic (HEABG) were investigated in human leukemic U937 cells.
MATERIALS/METHODS
Cytotoxicity was evaluated by MTT (3-(4, 5-dimethyl-thiazol-2-yl)-2, 5-diphenyl tetrazoliumbromide) assay. Apoptosis was detected using 4,6-diamidino-2-phenyllindile (DAPI) staining, agarose gel electrophoresis and flow cytometry. The protein levels were determined by Western blot analysis. Caspase activity was measured using a colorimetric assay.
RESULTS
Exposure to HEABG was found to result in a concentration- and time-dependent growth inhibition by induction of apoptosis, which was associated with an up-regulation of death receptor 4 and Fas legend, and an increase in the ratio of Bax/Bcl-2 protein expression. Apoptosis-inducing concentrations of HEABG induced the activation of caspase-9, an initiator caspase of the mitochodrial mediated intrinsic pathway, and caspase-3, accompanied by proteolytic degradation of poly(ADP-ribose)-polymerase. HEABG also induced apoptosis via a death receptor mediated extrinsic pathway by caspase-8 activation, resulting in the truncation of Bid, and suggesting the existence of cross-talk between the extrinsic and intrinsic pathways. However, pre-treatment of U937 cells with the caspase-3 inhibitor, z-DEVD-fmk, significantly blocked the HEABG-induced apoptosis of these cells, and increased the survival rate of HEABG-treated cells, confirming that HEABG-induced apoptosis is mediated through activation of caspase cascade.
Leukemia, a malignant hematopoietic tumor, is a cancer of the blood or bone marrow which is characterized by the abnormal proliferation of white blood cells, and is the sixth most common form of human cancer worldwide [1,2]. A combination of radiotherapy and adjuvant chemotherapy is now the standard treatment for leukemia, and these treatment modalities have been successful for certain patients. However, leukemia is highly resistant to radiotherapy and chemotherapy, and there is still no effective cure for patients in advanced stages of the disease [3,4]. Hence, novel therapeutic strategies are needed for more effective treatment of this serious and prevalent disease. New strategies which also inflict less morbidity and discomfort on the patient population are also desirable, as treatments which are better tolerated and more effective.
Because imbalance towards cell survival can result in cancer development and resistance to anticancer therapies such as radiotherapy and chemotherapy, current cancer therapy primarily induces apoptosis in cancer cells to prevent their development and progression [5]. Apoptosis is a highly regulated cell death mechanism, which is activated in response to various intra-/extracellular stimuli which damage cellular macromolecules or signals. Two major pathways for the induction of apoptosis have been identified, namely the mitochondrial mediated intrinsic-, and the death receptor mediated extrinsic apoptotic pathways [6,7]. The former is initiated through the release of signal factors, including cytochrome c by mitochondria within the cell, along with caspase-9 activation, which, in turn, activates caspase-3, ultimately resulting in the degradation of activated caspase-3 substrate proteins [8,9]. The intrinsic pathway is also regulated by the interplay between members of the Bcl-2 protein family, including both anti-apoptotic and pro-apoptotic members [10]. On the other hand, the latter is activated through ligand binding to death receptors on the cell surface, with sequential activation of caspase-8. Active caspase-8 activates downstream caspases, including caspase-3, and cleaves Bid, a pro-apoptotic Bcl-2 family member [11]. Truncated Bid (tBid) induces mitochondrial cytochrome c release. Therefore, the intrinsic or extrinsic pathways could be activated to amplify the apoptotic signal through cross-talk between the two pathways in different circumstances [12,13].
Many epidemiological studies have revealed a decreased incidence of various types of cancer in individuals who consume large amounts of fruits and vegetables [14]. Among them, garlic (Allium sativum) is a plant commonly used for seasoning food in many different cultures of the world, especially in Asian countries. Recently, extensive research has been carried out on the health promoting properties of garlic, and its importance as a functional food against various pathologies has been explored [15]. Although garlic derivatives have various biological properties, many people cannot easily eat fresh raw garlic because of its intense taste and smell. Moreover, the consumption of fresh raw garlic has been associated with several health hazards, such as stomach and digestion problems [16]. Therefore, different formulations of garlic have been developed. One of the most useful garlic preparations is aged black garlic, which is produced by aging whole garlic at high temperature and humidity [17]. Previous studies have shown that the extracts of aged black garlic have many pharmacological activities, including antioxidant, radical scavenging and anti-cancer effects [17,18,19,20,21,22]. Recently, Kim et al. [23] reported that a hexane extract of aged black garlic (HEABG) potently suppressed the proliferation of tumor necrosis factor (TNF)-α-activated human endometrial stromal cells, through inhibiting the activation of nuclear factor-κB (NF-κB) and activator protein-1 (AP-1) transcription factors. The results indicated that extracts of aged black garlic may be effective in the prevention and treatment of endometriosis in humans.
In this study, as a part of our on-going screening program to evaluate the anti-cancer potentials of aged black garlic, we investigated the pro-apoptotic properties of HEABG and the responsible underlying molecular mechanisms involved in human leukemic U937 cells. Our results indicated that HEABG induced apoptotic cell death and the effect is mediated through a signaling cascade of extrinsic as well as intrinsic caspase pathways.
U937 cells were obtained from the American Type Culture Collection (Manassas, VA). Cells were grown and maintained in RPMI 1640 media (GIBCO-BRL, Grand Island, NY) supplemented with 1% penicillin-streptomycin and 10% fetal bovine serum (FBS, GIBCO-BRL) at 37℃ in a humidified atmosphere of 95% air and 5% CO2. HEABG was isolated as previously described [20]. Briefly, aged black garlic extracts (500 ml) were purchased from Newgreen Food Co. (Changnyeong-gun, Gyeongsangnam-do, Republic of Korea) and successively partitioned with hexane, chloroform and n-butanol. The upper suspension layer was filtered and evaporated under reduced pressure at 45℃ and then lyophilized. A yellow, oily residue of hexane extract (17.6 mg) was obtained. The remaining aqueous layer was then sequentially partitioned with chloroform and n-butanol to yield chloroform (348 mg) and n-butanol fractions (11.53 g), respectively. The dried hexane fraction was used in this study and a 100 mg/mL concentration stock solution was made by dissolving in dimethyl sulfoxide (DMSO, Sigma-Aldrich Chemical Co., St Louis, MO), and then diluted with the medium to the desired concentration prior to use. The morphological appearance of HEABG-treated cells was then compared with that of the untreated control, as observed under phase contrast inverted microscope.
The effect of HEABG on cell proliferation was determined by MTT [3-(4, 5-dimethyl-thiazol-2-yl)-2, 5-diphenyl tetrazoliumbromide, Sigma-Aldrich] assay, and growth inhibition was assessed as the percent viability, where vehicle-treated cells were taken as 100% viable, as previously described [24].
To detect apoptosis, nuclear staining was performed. Briefly, after HEABG treatment for 24 h, cells were harvested, washed with cold phosphate-buffered saline (PBS), and fixed with 3.7% paraformaldehyde in PBS for 10 min at room temperature. The cells were then stained with a 4,6-diamidino-2-phenyllindile (DAPI, Sigma-Aldrich) solution at room temperature for 10 min, and washed twice more with PBS. The morphologic changes were observed using a fluorescence microscope (Carl Zeiss, Oberkochen, Germany).
For the detection of DNA fragmentation, cells were lysed in a solution (10 mM Tris-HCl pH 7.4, 150 mM NaCl, 5 mM EDTA, and 0.5% Triton X-100) at room temperature for 20 min. The lysates were vortexed and cleared by centrifugation at 14,000 rpm for 20 min. The DNA in the supernatant was extracted using a 25:24:1 (v/v/v) equal volume of neutral phenol: chloroform: isoamyl alcohol and analyzed electrophoretically on 1.0% agarose gels containing 0.1 µg/ml ethidium bromide (EtBr, Sigma-Aldrich).
For cell cycle analysis, cells were collected, washed, and resuspended in propidium iodide (PI, Sigma-Aldrich) and 100 µg/ml RNase in PBS. The samples were then incubated at room temperature for 30 min, and flow cytometry was performed using a flow cytometer (Becton Dickinson, SanJose, CA) and analyzed using Cell Quest Analysis software 'Modfit' to determine the number of cells in each phase of the cell cycle. Fluorescence intensity of the sub-G1 cell fraction represents the apoptotic cell population.
The cells were harvested, lysed, and the protein concentration in the cell lysate was determined using detergent-compatible protein assay from Bio-Rad (Hercules, CA), according to the procedure of the manufacturer. Equivalent amounts of proteins were separated by electrophoresis on sodium dodecyl sulfate (SDS)-polyacrylamide gels, followed by transferring to nitrocellulose membranes (Schleicher & Schuell, Keene, NH). After blocking with 5% skimmed milk, the blot was incubated with protein specific antibodies for 1 h, and was subsequently incubated with appropriate enzyme-linked secondary antibodies and visualized by enhanced chemiluminescence (ECL, Amersham Corp., Arlington Heights, IL) according to the recommended procedure. Antibodies were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA), Invitrogen Corp. (Carlsbad, CA) and Sigma-Aldrich. Peroxidase-labeled donkey antirabbit immunoglobulin and peroxidase-labeled sheep antimouse immunoglobulin were purchased from Amersham Corp.
To evaluate caspase activity, caspase colorimetric protease assay kits (Millipore, Billerica, MA) were used. In brief, harvested cell pellets were lysed in the lysis buffer, and supernatants were collected. Equal amounts of protein were incubated with reaction buffer and colorimetric substrate, acetyl (Ac)-Ile-Glu-Thr-Asp (IETD) p-nitroaniline (pNA) for caspase-8, Ac-Leu-Glu-His-Asp (LEHD)-pNA for caspase-9, and Ac-Asp-Glu-Val-Asp (DEVD)-pNA for caspase-3, respectively, at 37℃ for 2 h in the dark. Thereafter, the reactions were assessed for changes in absorbance at 405 nm using a microplate reader. In the caspase-3 inhibitor assay, cells were pretreated with a caspase-3 inhibitor (50 µM zDEVD-fmk) for 1 h prior to the addition of HEABG [25].
U937 cells were exposed to various concentrations of HEABG for 24 h or 10 µg/ml of HEABG for the indicated times. The MTT assay was used to examine the effect of HEABG on cell viability. Results showed that the viability of HEABG-treated cells decreased in time- and concentration-dependent manners (Fig. 1A and B), demonstrating that HEABG has a cytotoxic effect on U937 cells. Consistent with these effects, after treatment with HEABG, U937 cells showed changes in morphology, including blebbing of the cell membrane and shrinkage of the cells, in a time-dependent manner.
To elucidate whether HEABG inhibits cell growth through the induction of apoptosis, we investigated the effects of HEABG on apoptosis in U937 cells using different approaches. As shown in Fig. 2A, DAPI staining showed relatively many apoptotic bodies containing nuclear fragments in cells treated with HEABG in a concentration-dependent manner, but few were observed in cells treated with vehicle. Besides cell morphology, DNA fragmentation in the HEABG-treated cells was confirmed by agarose gel electrophoresis, which showed the presence of DNA laddering, a marker of apoptosis, in HEABG-treated U937 cells, whilst the untreated control did not show evidence of ladders (Fig. 2B). In addition, using flow cytometric analysis to detect increases in hypodiploid sub-G1 cell populations, HEABG increased the percentage of the sub-G1 population in U937 cells, reaching 18.2% and 25.0% after 24 h of treatment with HEABG at 7.5 µg/ml and 10 µg/ml, respectively (Fig. 2C). Thus, these results indicate that HEABG triggered apoptosis in U937 cells, as the chromosomal DNA cleavage into oligonucleosomal size fragments is an integral part of apoptosis induction.
To evaluate the molecular mechanism by which HEABG induced apoptosis in U937 cells, we examined the expression of several apoptosis-associated proteins, including death receptor (DR)-related and Bcl-2 family proteins, by immunoblotting. The results of Western blot analysis showed that HEABG treatment increased the levels of DR4 and Fas legend (FasL) in a concentration-dependent manner, but did not affect the expression of TNF-related apoptosis, inducing ligand (TRAIL), DR5 and Fas (Fig. 3). Furthermore, we found that HEABG treatment resulted in a decrease in anti-apoptotic Bcl-2 expression with a concomitant increase in the protein levels of pro-apoptotic Bax (Fig. 3), resulting in a substantial increase in the Bax/Bcl-2 ratio, which favors apoptosis. We also observed that HEABG treatment of U937 cells truncated Bid (tBid) expression, which is well known as a linker between the endogenous mitochondrial intrinsic pathway and death receptor mediated extrinsic apoptotic pathway. This observation suggests that a cross-talk exists between intrinsic and extrinsic pathways in HEABG-induced U937 cell apoptosis.
Caspases are important regulators of cell apoptosis. Caspases -8 and -9 are the initiator caspases of extrinsic and intrinsic pathways, respectively, while caspase-3 is an executioner caspase. To better characterize the pathway through which HEABG exerts its apoptotic effects in U937 cells, we examined the effects of HEABG on the levels and activities of these caspases. The results shown in Fig. 4 indicate that HEABG significantly increased the cleaved levels of caspase-9, -8 and -3, as well as the in vitro activities of these caspases, in a concentrationdependent manner, indicating that the induction of apoptosis occurred through both extrinsic and intrinsic pathways. In addition, a significant concentration-dependent increase in cleaved poly (ADP-ribose) polymerase (PARP), a substrate of caspase 3, which is considered as an important biomarker of apoptosis, was also observed in cells treated with HEABG (Fig. 4A).
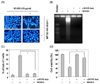
To investigate if apoptosis upon HEABG treatment was induced via activation of caspase-3, cells were treated with a caspase-3 inhibitor, z-DEVD-fmk, for 2 h, followed by treatment with HEABG for an additional 24 h. Fig. 5 shows that blockage of caspase-3 activation by z-DEVD-fmk completely abrogated PARP degradation induced by HEABG, and that z-DEVD-fmk attenuated HEABG-induced apoptotic features such as chromatin condensation, DNA fragmentation and accumulation of sub-G1 cells in U937 cells (Fig. 6A-C). Furthermore, cell viability was markedly recovered in HEABG-treated cultures where z-VAD-fmk was included (Fig. 6D), suggesting that apoptosis is essentially induced through via a caspase-3-dependent pathway.
Dysregulation of proliferation and apoptosis are linked to the development of most cancers. Therefore, the ability of cancer cells to induce the apoptotic program has been identified as one of the major mechanisms which might serve for the development of novel approaches to treat cancer. Aged black garlic has been generally reported to have excellent antioxidant potency when compared to fresh or cooked garlic. The reason is probably that the bioactivity of some natural products increases during the process of fermentation [17,18,19,20,21,22,23], but no specific experimental proof has yet established its anti-cancer efficacy. In this study, we have demonstrated that HEABG significantly decreased cell proliferation in human leukemic U937 cells by inducing apoptosis, as determined by DAPI staining, agarose gel electrophoresis and flow cytometric analyses.
Among two apoptosis pathways, the intrinsic pathway is mainly regulated by the members of the Bcl-2 family proteins [6,7]. The anti-apoptotic proteins of this family, such as Bcl-2 and Bcl-xL promote cell survival by inhibiting the mitochondrial permeability transition and cytochrome c release, thereby functioning to block apoptosis, whereas pro-apoptotic proteins, including Bax and Bad, promote cell death through reduction of the mitochondrial membrane potential [9,10]. Therefore, the ratio of pro- to anti-apoptotic molecules is considered to be a determinant for mitochondria-related apoptosis. In the present study, we observed that HEABG significantly down-regulated Bcl-2 protein and up-regulated levels of Bax protein in U937 cells (Fig. 3), suggesting the involvement of an intrinsic apoptosis pathway by which HEABG induces apoptosis in U937 cells.
In general, caspases play important roles in apoptosis triggered by various proapoptotic signals. Activation of the caspase-dependent cascade requires both initiator caspases, such as caspase-8 and -9, and effector caspases, such as caspase-3. The effector caspases cleave several vital substrates leading to apoptosis [8,9]. In our findings, HEABG treatment activated caspase-9, an initiator caspase of the intrinsic apoptosis pathway, and caspase-3, and induced a concentration-dependent progressive proteolytic degradation of PARP, a substrate of activated caspase-3 (Fig. 4), indicating that HEABG-induced apoptosis of U937 cells is involved in the intrinsic executioner pathway. Of note, in addition to the activation of caspase-9, we also found that HEABG activated caspase-8, an initiator caspase of the extrinsic apoptosis pathway, and increased the levels of cleaved Bid (tBid). Bid, a BH3 domain-containing a pro-apoptotic Bcl-2 family member, is a specific substrate of caspase-8 in the extrinsic apoptotic signaling pathway [11]. The possibility that caspase-8 activation by HEABG is a death receptor-mediated event, or alternatively represents a secondary event derived from mitochondrial activation. Our results demonstrated that HEABG increased expression of DR4 and FasL at protein levels, but did not affect the expression of DR5, TRAIL or Fas in U937 cells (Fig. 3). HEABG is well known as a linker between the intrinsic pathway and the extrinsic apoptotic pathway. Full-length Bid is inactive and localized in the cytosol, while tBid translocates to the mitochondria and transduces apoptotic signals from the cytoplasm to the mitochondria, increasing mitochondrial membrane permeabilization and the release of apoptosis-associated mitochondrial proteins [11,12]. Although further studies are needed, these data suggest the possibility of cross-talk between the two pathways, as activated caspase-8 can cleave Bid to produce t-Bid. Moreover, the cell-permeable caspase inhibitor z-DEVD-fmk, a caspase-3 specific inhibitor, suppressed HEABG-induced apoptosis, which was accompanied by the marked attenuation of HEABG-induced growth inhibition (Fig. 5 and 6). Therefore, our data provide evidence that HEABG induces caspase-dependent apoptosis of U937 cells via a signaling cascade of death-receptor-mediated extrinsic as well as mitochondria-mediated intrinsic caspase pathways.
Based on our findings, we propose a HEABG-induced apoptosis signaling pathway in human leukemic U937 cells. First, the death receptor-mediated extrinsic pathway is initiated by ligation of the transmembrane death receptor to activate membrane-proximal caspases (caspase-8), which, in turn, activates effector caspases, such as caspases-3. Second, the mitochondrial-mediated intrinsic pathway requires disruption of the mitochondrial membrane, which induces activation of caspase-9, and thereby initiates the apoptotic caspase cascade. In addition, cross talk between the two pathways is mediated by tBid, which may act as a potential feedback loop to amplify HEABG-induced caspase-dependent apoptosis. Although all of the experiments here were performed in just one cell line, and further in vivo studies are needed, these findings warrant further investigation of aged black garlic as a source of anti-cancer agents, and underline the necessity for identification and purification of active compounds in the present HEABG.
Figures and Tables
Fig. 1
Effect of HEABG on the viability and morphology of U937 cells. (A and B) Cells were cultured with the indicated concentration of HEABG for 24 h (A) or with 10 µM HEABG for the indicated times (B). Cell viability was measured by MTT assay. Each point is the mean ± SD of three independent experiments. The significance was determined using Student's t-test (*P < 0.05 vs. untreated control). (C) The morphological changes of U937 cells treated with the indicated concentration of HEABG for 24 h were visualized by phase-contrast microscopy (200×).

Fig. 2
Induction of apoptosis by HEABG in U937 cells. Cells were treated with the indicated concentration of HEABG for 24 h. (A) Cells were fixed, stained with DAPI solution, and examined by fluorescence microscopy (400×). (B) DNA fragmentation was examined by 1.0% agarose gel electrophoresis of genomic DNA, followed by EtBr staining. (C) To quantify the degree of apoptosis induced by HEABG, cells were evaluated using a flow cytometer for sub-G1 DNA content, which represents the cells undergoing apoptotic DNA degradation. Each point represents the mean ± SD of three independent experiments. Significance was determined using Student's t-test (*P < 0.05 vs. untreated control).
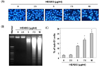
Fig. 3
Effect of HEABG on the expression of apoptosis-associated proteins in U937 cells. Cells were treated with the indicated concentration of HEABG for 24 h. Cell lysates were resolved by SDS-polyacrylamide gels and subjected to Western blotting. The proteins were visualized using specific antibodies and an ECL detection system. Actin was used as an internal control. The expression of the indicated proteins were measured by densitometry and expressed as relative ratio. The results were representatives of two independent experiments.

Fig. 4
Activation of caspases and the degradation of PARP by HEABG in U937 cells. (A) Cells were treated with the indicated concentrations of HEABG for 24 h. The cells were lysed, and the cellular proteins were then separated by SDS-polyacrylamide gels and transferred onto nitrocellulose membranes. The proteins were visualized using the indicated antibodies and an ECL detection system. Actin was used as an internal control. (B) After 24 h of incubation with the indicated concentrations of U937, the cells were lysed, and aliquots (50 µg protein) were assayed for in vitro caspase-3, -8, and -9 activity using DEVD-pNA, IETD-pNA and LEHD-pNA as substrates, respectively, at 37℃ for 1 h. The released fluorescent products were measured. Each point represents the mean ± SD of three independent experiments. Significance was determined using Student's t-test (*P < 0.05 vs. untreated control).
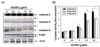
Fig. 5
Inhibition of PARP degradation by caspase-3 inhibitor in HEABG-treated U937 cells. (A) Cells were incubated with 10 µg/ml HEABG for 24 h after 1 h pretreatment with z-DEVD-fmk (50 µM). Cell lysates were resolved by SDS-polyacrylamide gels and subjected to Western blotting. The proteins were visualized using the anti-caspase-3 and anti-PARP antibodies, and an ECL detection system. (B) Cells grown under the same conditions as (A) were assayed for in vitro caspase-3 activity using DEVD-pNA as substrates. Each point represents the mean ± SD of three independent experiments. Significance was determined using Student's t-test (*P < 0.05 vs. HEABG-treated cells).

Fig. 6
Caspase-3 inhibitor alleviates HEABG-induced apoptosis in U937 cells. (A) Cells grown under the same conditions as Fig. 5 were fixed and stained with DAPI solution. The stained nuclei were observed under a fluorescent microscope (original magnification 400×). (B) DNA fragmentation was examined by 1.0% agarose gel electrophoresis of genomic DNA, followed by EtBr staining. (C) Cells were evaluated by flow cytometry for sub-G1 DNA content, suggestive of apoptotic cell death. (D) Cell viability was determined by MTT assay. Each point represents the mean ± SD of three independent experiments. The significance was determined using Student's t-test (*P < 0.05 vs. HEABG-treated cells).
References
1. Abramson N, Melton B. Leukocytosis: basics of clinical assessment. Am Fam Physician. 2000; 62:2053–2060.
2. Gilliland DG, Jordan CT, Felix CA. The molecular basis of leukemia. Hematology Am Soc Hematol Educ Program. 2004; 80–97.

3. Voliotis D, Diehl V. Challenges in treating hematologic malignancies. Semin Oncol. 2002; 29:30–39.

4. Leone G, Fianchi L, Voso MT. Therapy-related myeloid neoplasms. Curr Opin Oncol. 2011; 23:672–680.

5. Cotter TG. Apoptosis and cancer: the genesis of a research field. Nat Rev Cancer. 2009; 9:501–507.

6. Jin Z, El-Deiry WS. Overview of cell death signaling pathways. Cancer Biol Ther. 2005; 4:139–163.

8. Lazebnik YA, Kaufmann SH, Desnoyers S, Poirier GG, Earnshaw WC. Cleavage of poly(ADP-ribose) polymerase by a proteinase with properties like ICE. Nature. 1994; 371:346–347.

10. Kutuk O, Letai A. Regulation of Bcl-2 family proteins by posttranslational modifications. Curr Mol Med. 2008; 8:102–118.

11. Lee EW, Seo J, Jeong M, Lee S, Song J. The roles of FADD in extrinsic apoptosis and necroptosis. BMB Rep. 2012; 45:496–508.

13. Lee EN, Choi YW, Kim HK, Park JK, Kim HJ, Kim MJ, Lee HW, Kim KH, Bae SS, Kim BS, Yoon S. Chloroform extract of aged black garlic attenuates TNF-α-induced ROS generation, VCAM-1 expression, NF-κB activation and adhesiveness for monocytes in human umbilical vein endothelial cells. Phytother Res. 2011; 25:92–100.

14. Dolara P, Bigagli E, Collins A. Antioxidant vitamins and mineral supplementation, life span expansion and cancer incidence: a critical commentary. Eur J Nutr. 2012; 51:769–781.

15. Thomson M, Ali M. Garlic [Allium sativum]: a review of its potential use as an anti-cancer agent. Curr Cancer Drug Targets. 2003; 3:67–81.

16. Butt MS, Sultan MT, Butt MS, Iqbal J. Garlic: nature's protection against physiological threats. Crit Rev Food Sci Nutr. 2009; 49:538–551.

17. Imai J, Ide N, Nagae S, Moriguchi T, Matsuura H, Itakura Y. Antioxidant and radical scavenging effects of aged garlic extract and its constituents. Planta Med. 1994; 60:417–420.

18. Lee YM, Gweon OC, Seo YJ, Im J, Kang MJ, Kim MJ, Kim JI. Antioxidant effect of garlic and aged black garlic in animal model of type 2 diabetes mellitus. Nutr Res Pract. 2009; 3:156–161.

19. Kim HK, Choi YW, Lee EN, Park JK, Kim SG, Park DJ, Kim BS, Lim YT, Yoon S. 5-Hydroxymethylfurfural from black garlic extract prevents TNFα-induced monocytic cell adhesion to HUVECs by suppression of vascular cell adhesion molecule-1 expression, reactive oxygen species generation and NF-κB activation. Phytother Res. 2011; 25:965–974.

20. Jeong YY, Park HJ, Cho YW, Kim EJ, Kim GT, Mun YJ, Lee JD, Shin JH, Sung NJ, Kang D, Han J. Aged red garlic extract reduces cigarette smoke extract-induced cell death in human bronchial smooth muscle cells by increasing intracellular glutathione levels. Phytother Res. 2012; 26:18–25.

21. Colín-González AL, Santana RA, Silva-Islas CA, Chánez-Cárdenas ME, Santamaría A, Maldonado PD. The antioxidant mechanisms underlying the aged garlic extract- and S-allylcysteine-induced protection. Oxid Med Cell Longev. 2012; 2012:907162.

22. Wang X, Jiao F, Wang QW, Wang J, Yang K, Hu RR, Liu HC, Wang HY, Wang YS. Aged black garlic extract induces inhibition of gastric cancer cell growth in vitro and in vivo. Mol Med Rep. 2012; 5:66–72.
23. Kim KH, Park JK, Choi YW, Kim YH, Lee EN, Lee JR, Kim HS, Baek SY, Kim BS, Lee KS, Yoon S. Hexane extract of aged black garlic reduces cell proliferation and attenuates the expression of ICAM-1 and VCAM-1 in TNF-α-activated human endometrial stromal cells. Int J Mol Med. 2013; 32:67–78.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download