Abstract
Synurus deltoides (Aiton) Nakai, belonging to the Compositae family, is an edible plant widely distributed in Northeast Asia. In this study, we examined the mechanisms underlying the immunomodulative effects of the ethanol extract of S. deltoides (SDE). The SDE extract strongly down-regulated the mRNA expression of the inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2), and tumour necrosis factor (TNF)-α, thereby inhibiting the production of nitric oxide (NO), prostaglandin E2 (PGE2), and TNF-α in the lipopolysaccharide (LPS)-stimulated RAW 264.7 cells. Furthermore, SDE also suppressed the nuclear translocation of the activation protein (AP)-1 and the nuclear factor-κB (NF-κB), and simultaneously decreased the phosphorylation of extracellular signal-regulated protein kinases (ERK), p38, and Akt. In agreement with the in vitro observations, the orally administered SDE ameliorated the acute inflammatory symptoms in the arachidonic acid-induced ear edema and the EtOH/HCl-induced gastritis in mice. Therefore, S. deltoides have a potential anti-inflammatory capacity in vitro and in vivo, suggesting the potential therapeutic use in the inflammation-associated disorders.
Inflammatory responses are recognized as the natural defence mechanisms that are critical for the recruitment of a variety of immune cells and molecules to the sites with the infectious microbes or the injured tissues [1]. Acute inflammation is a limited beneficial process, particularly in response to the infectious pathogens, whereas the chronic inflammation is an undesirable persistent phenomenon that can ultimately result in the development of inflammatory diseases [2,3]. Inflammation is an innate immune event that is primarily mediated by a variety of immune cells [4]. The inflammatory macrophages are generally induced by immunogens, which include various inflammatory molecules and inflammatory mediators that interact with the pattern-recognition receptors and their adaptor molecules [5]. Macrophages play a central role in producing the soluble factors, such as nitric oxide (NO), cyclooxygenase-2 (COX-2), and prostaglandin E2 (PGE2), as well as some cytokines in response to the extracellular stimuli including bacterial lipopolysaccharide (LPS) [6,7]. These factors are primarily controlled by the surface molecules, such as the pattern-recognition receptors (e.g., toll-like receptor 4) and their counter-adaptor molecules, which include TANK binding kinase 1, Toll-IL-1 receptor-domain-containing adapter-inducing interferon-β (TRIF), TRIF-related adaptor molecule (TRAM), and myeloid differentiation primary response gene 88 (MyD88) [8]. These inflammatory events require functional upregulation of the intracellular signaling machinery, including the levels of transcription factors and the upstream signaling cascades [9]. Thus, the suppression of the targets specific to the inflammatory process may have a great potential for preventing and treating the inflammation-mediated diseases.
Synurus deltoides (Aiton.) Nakai (Compositae) is wildly distributed in the mountainous areas and is one of the edible green-hued plants used as a natural coloring-agent for the rice cakes [10]. The plant has been used in the traditional medicine system to treat cystitis, bleeding, vomiting, hematemesis, and edema [11]. Several chemical compounds (e.g., anthocyanins, 20-hydroxyecdysone, terpenoids, coumarins, flavonoids, and triterpenoids) have been isolated from S. deltoides [12-15]. Despite the occurrence of some preliminary published works describing a variety of its pharmacological activities, including the antioxidant, antimutagenicity, and anti-inflammatory activities [16-18], the precise molecular mechanisms underlying the anti-inflammatory properties of the plant have not been fully investigated. In this study, we aimed to elucidate the anti-inflammatory activities of the ethanol extract of S. deltoides (SDE), using the LPS-activated macrophages and the acute in vivo inflammatory models.
The dried powder of S. deltoides leaves (10 kg) was extracted three-times with ethanol at room temperature for 24 h. Following the drying process, by the evaporation of water using a vacuum rotary evaporator, a crude extract of 974.58 g was produced. A voucher specimen (No. 2011SD) was deposited in the molecular plant biotechnology lab. The supplies of sulfanilamide, naphthylethylenediamine dihydrochloride, 2',7'-dichlorodihydrofluorescein diacetate (DCFH-DA), LPS (E.coli 0111 : B4), phorbal-12-myristate-13-acetate (PMA), and 1-(4,5-dimethylthiazol-2-yl)-3,5-diphenylformazan (MTT) were purchased from Sigma (St. Louis, MO, USA). The kit for RNA isolation and the first-strand cDNA synthesis were obtained from Invitrogen (Carlsbad, CA). The RPMI medium 1640, Dulbecco's modified Eagle's medium (DMEM), trypsin-EDTA, and fetal bovine serum (FBS) were acquired from Gibco BRL (Grand Island, NY, USA). All culture supplies were obtained from the BD-Falcon brand (BD, Franklin Lakes, NJ). The phospho-specific ERK, c-Jun N-terminal kinase (JNK), IκBα, p38 mitogen-activated protein kinase (p38 MAPK), and the total antibodies to ERK, JNK, IκBα, p38 MAPK, and β-actin were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The antibodies against NF-κB, c-Fos, and c-Jun were purchased from Cell Signaling (Beverly, MA, USA). Antibody-binding was detected with WEST-SAVE Up™ enhanced chemiluminescence (ECL) Western blotting substrate (AbFrontiers, Suwon, Korea). All other chemicals were of the analytical grade.
The RAW 264.7 cells and the human embryonic kidney cells (HEK 293) were purchased from the Korean Cell Line Bank (Seoul, Korea). The RAW 264.7 cells were maintained in RPMI 1640, supplemented with 10% FBS, 100 U/ml of penicillin, and 100 µg/ml of streptomycin. The HEK 293 cells were grown in DMEM, and supplemented with 10% FBS, 100 U/ml of penicillin, and 100 µg/ml of streptomycin. The cells were incubated at 37℃ in a humidified atmosphere of 95% air and 5% CO2.
The cytotoxicity of SDE on the RAW 264.7 cells was investigated. The cells were seeded into a 96-well plate at a density of 1 × 105 cells/well for 16 h and then exposed to the medium in the presence of different concentrations of SDE for 24 h. After removing the supernatant of each well, a total of 10 µl of the MTT solution [5 mg/ml in phosphate-buffered saline (PBS)] and 90 µl of FBS-free medium were added to each well at the time of incubation for 4 h at 37℃. The dark-blue formazan crystals formed inside the intact mitochondria were solubilized with 100 µl of MTT stop solution [containing 10% sodium dodecyl sulfate (SDS) and 0.01 M hydrochloric acid]. The amount of MTT formazan was qualified by measuring at 550 nm, using an enzyme-linked immunosorbent assay (ELISA) plate reader (ELx800TM, Bio-Tek, Winooski, VT, USA). The optical density of formazan formed in the control cells was taken as 100% viability. Cell viability was expressed as a percentage of the control culture value. Data were calculated as the percentage of inhibition by the following formula: Cell viability (%) = ODs/ODv × 100%. ODs and ODv indicated the optical density of cell lines incubated with SDE and vehicle control, respectively. Cytoprotective effect (%) = ODls/ODv × 100%. ODls and ODv indicated the optical density of cell lines incubated with SDE in present of LPS and vehicle control, respectively.
The RAW 264.7 cells were plated in a 96-well cell plate and stimulated with LPS (1 µg/ml) in the presence or absence of various concentration of SDE for 24 h. The aliquots of 100 µl of the cell culture medium were mixed with 50 µl of 1% sulfanilamide (in 5% phosphoric acid) and 50 µl of 0.1% naphthylethylenediamine dihydrochloride, at room temperature. The absorbance was determined at 550 nm using an ELISA plate reader (EL × 800TM). The levels of PGE2 and TNF-α were determined using the commercially available kits (Enzo Life Sciences, Farmingdale, NY), according to the manufacturer's instructions.
A total of 5 × 104 RAW 264.7 cells were plated per well in a 96-well plate for 16 h. The cells were pretreated with various concentrations of SDE for 30 min, before being stimulated with LPS (1 µg/ml) for 24 h. After incubation, the supernatant of each well was removed, and the cells were washed with a preheated PBS at 37℃. DCFH-DA (20 µM) was then added, and the cells were incubated for 30 min. DCFH-DA was then removed from each well, and 100 µl of cold PBS was added. Fluorescence intensity (485 nm/535 nm, ex/em) was measured using a fluorescence spectrophotometer (Victor 3, PerkinElmer, New York, USA).
RT-PCR was used to analyze the gene expression in the RAW 264.7 cells, following stimulation with LPS in the presence of different concentrations of SDE for 6 h. The total RNA was isolated with a Trizol reagent in accordance with the manufacturer's instructions (Invitrogen, Carlsbad, CA). The first-strand cDNA was synthesized from the total RNA (2 µg), containing oligo (dT) primers and Moloney murine leukemia virus reverse transcriptase (M-MLV RT, Invitrogen). The primer sequences for iNOS, COX-2, TNF-α, and GAPDH are listed in Table 1. The aliquots of the individual PCR products were separated on 1% agarose gel, stained with ethidium bromide, and imaged using a Mini BIS image analysis system (DNR Bio-Imaging Systems Ltd., Jerusalem, Israel). Densitometric analysis was done using an image analysis software (Quantity One; Bio-Rad, Hercules, CA, USA).
The HEK 293 cells (1×106 cells/ml) were transfected with 1 µg of plasmids containing AP-1-Luc or NF-κB as well as β-galactosidase, using the PEI method in the 12-well plates. After 24 h, the transfected cells were treated with different concentrations of SDE in the presence or absence of PMA. The RAW 264.7 cells (1×106 cells/ml) were transfected with 1 µg of plasmids containing AP-1-Luc or NF-κB as well as β-galactosidase, using the lipofectamine 2000 in the 12-well plates. After 24 h, the transfected cells were treated with different concentrations of SDE in the presence or absence of LPS. Luciferase assays were performed using the luciferase assay system (Promega, Madison, WI, USA).
The RAW 264.7 cells (1 × 106 cells) were incubated in a 6-well plate. After 16 h incubation, the cells were treated with SDE for the predetermined times. Nuclear and total protein extracts were prepared [19]. The concentration of protein was determined using the Bradford assay. The aliquots of the lysates (40 µg of protein) were boiled at 94℃ for 5 min and separated on a sodium dodecyl sulfate-polyacrylamide gel and transferred to the polyvinylidene difuoride (PVDF; Bio-Rad) membranes. The membranes were blocked in a blocking buffer (Tris-buffered saline containing 3% BSA, 20 mM NaF, 2 mM EDTA, and 0.2% Tween 20) for 60 min at room temperature. The membrane was incubated for 60 min with the appropriate primary antibody at room temperature, washed three times with the TBST buffer (Tris-buffered saline containing 20 mM NaF, 2 mM EDTA, and 0.2% Tween 20), further incubated for 60 min with the HRP-conjugated secondary antibody, and washed three times with a TBST buffer. The bound antibodies were detected by the ECL system. Bands were finally visualized by a Mini BIS image analysis system (DNR Bio-Imaging Systems Ltd., Jerusalem, Israel).
The ICR mice (6-8 weeks old, 17-21 g) were purchased from DAEHAN BIOLINK (Chungbuk, Korea) and housed at room temperature (24 ± 5℃), under relative humidity (50 ± 10%), with a 12 h light/12 h dark cycle. All animals were allowed free access to a commercial stock diet and water throughout the experiment. Studies were performed in accordance with the guidelines established by the Kangwon University Institutional Animal Care and Use Committee (KIACUC-12-0018).
The ICR mice (n = 6) were orally pre-treated with SDE (40 mg/kg) or indomethacin (1 mg/kg) for 3 days. After the final treatment, arachidonic acid [2% (w/v)] was applied to the ear of the mouse (30 µl/ear). The ear thicknesses were measured using a dial thickness gauge (Mitutoyo, Japan), 1 h after the arachidonic acid treatment.
The inflammation of the stomach in mice was induced with EtOH/HCl according to a published method [20]. The fasted ICR mice (n = 6) were orally treated with SDE (100 mg/kg) or ranitidine (40 mg/kg), twice per day for 3 days. Thirty minutes after the final oral administration of SDE or ranitidine, the mice were orally treated with 0.3 ml of 60% ethanol in 150 mM HCl. Each animal was anesthetized with an overdose of ether, 1 h after the post-administration of EtOH/HCl. The stomach was excised, gently rinsed under the running tap water, inflated by an injection of saline, and then fixed for 30 min in 5% formalin. After opening the stomach along the greater curvature and spreading it out on a board, the area (mm2) of the mucosal erosive lesion was measured using a pixel-counter under a blinded condition. Ranitidine was used as a positive control drug.
All tests were carried out independently in triplicates (n = 3). The data are expressed as the mean ± standard deviation (SD). One-way analysis of variance (ANOVA) was used to determine the significant differences between the groups, followed by a Dunnett's t-test for multiple comparisons. A probability < 0.05 was considered as significant. All analyses were performed using SPSS 16 (SPSS Institute, Cary, NC, USA).
The cytotoxic effects of SDE were presented in Fig. 1A. The results showed that SDE alone at 100, 150, 200, 300, and 400 µg/ml did not exhibit any toxicity in the RAW 264.7 cells. SDL reduced cell viability to 68.54 ± 6.53% at 600 µg/ml (data not shown). Stimulation with LPS for 24 h led to a robust increase in the NO, PGE2 and TNF-α production. However, SDE significantly suppressed NO, PGE2, and TNF-α by the LPS-stimulated RAW 264.7 cells (Fig. 1B-D). Furthermore, this extract strongly prevented cells from the LPS-induced cytotoxicity at high concentrations (Fig. 1E).
The levels of the ROS production in the RAW 264.7 cells were determined using a DCFH-DA fluorescence probe and a fluorescence spectrophotometer. As shown in Fig. 2, the ROS levels in the macrophages increased significantly to 532.40% when the RAW 264.7 cells were exposed to LPS, compared to the control without LPS. The pre-treatment with various concentrations of SDE rapidly suppressed ROS in the RAW 264.7 cells.
The iNOS, COX-2 and TNF-α mRNA expression in the unstimulated RAW 264.7 cells were undetectable; however, their mRNAs were profoundly induced after the treatment with LPS. Pretreatment with SDE markedly suppressed the LPS-stimulated iNOS, COX-2 and TNF-α expression (Fig. 3).
As shown in Fig. 4, SDE significantly interrupted c-Jun, c-fos and p65 translocation to the nuclear fraction, without contaminating the cytosolic proteins, as confirmed by cytosolic β-tubulin (data not shown). Phorbol myristic acid (PMA) and LPS upregulated luciferase activity in the HEK 293 cells and RAW 264.7 cells, respectively, which were transfected with a luciferase reporter construct containing AP-1 or NF-κB binding sites. There were also suppressed by the SDE treatment (Fig. 5A-D).
As shown in Fig. 6A, at an early time point (5 min), the phosphorylation of p38 was suppressed by SDE. The ERK phosphorylation at 15 and 30min was also reduced by the SDE extract. However, SDE did not affect JNK phosphorylation. As shown in Fig. 6B, SDE blocked IκBα phosphorylation and Akt phosphorylation, an essential step for the NF-κB translocation. Furthermore, the phosphorylation of the upstream signaling kinase [p85 (a regulatory subunit of PI3K)] did not decrease following the SDE treatment (data not shown).
As shown in Fig. 7, ear edema induced by the arachidonic acid treatment was significantly suppressed by SDE as well as in the positive control (indomethacin). However, the oral administration of indomethacin severely reduced the normal body weight, whereas SDE did not (data not shown).
As shown in Fig. 8, the oral administration of EtOH/HCl dramatically increased the prevalence of the inflamed lesions in the stomach of mouse. The SDE (100 mg/kg) extract markedly reduced the gastric damage induced by EtOH/HCl to a level similar to the treatment with ranitidine (40 mg/kg).
Inflammation is generally recognized as a cause of various diseases, such as cancer, diabetes, atherosclerosis, sepsis, and obesity. The inflammatory processes are mediated by multiple molecular mechanisms [21]. Thus, the RAW264.7 macrophages provide a useful model for evaluating the anti-inflammatory agents. A number of different inflammatory mediators including NO, PGE2 and TNF-α are generated by the macrophages upon stimulation with LPS (a primary component of the Gram-negative bacteria cell wall) and plays an important role in the immunepathology of the acute or chronic inflammatory diseases [22,23]. This observation could lead to the development of novel anti-inflammatory drugs without side-effects, aimed at preventing such diseases. First, the viability was tested at various SDE concentrations in the cells by the mitochondrial reduction of MTT, and these values were expressed as a percentage of the viability with respect to the control group (100% viability) (Fig. 1A). Based on these results, the SDE concentrations less than 400 µg/ml were selected for further studies. The cells were treated with different concentrations of SDE for 30 min, followed by a 24 h treatment with LPS, NO, and PGE2. The TNF-α levels in the cell supernatants were determined by the Griess assay and an ELISA kit.
Mammalian cells are constantly exposed to ROS as a result of normal metabolic processes occurring during aerobic respiration; however, excessively high levels of free radicals or ROS generate oxidative stress lead to enhanced lipid peroxidation and oxidative stress in cells. The macrophages stimulated with LPS generate ROS via activation of a membrane-bound NADPH oxidase [24,25]. Therefore, SDE acts as a potential ROS scavenger in an oxidative environment to balance the ROS levels and may inhibit the cytotoxicity induced by LPS.
It remains unclear whether the SDE-mediated inhibition of NO, PGE2, and TNF-α is the consequence of inhibiting iNOS, COX-2, and TNF-α at the transcriptional level or due to some other mechanisms. Our studies were extended to determine the iNOS, COX-2, and TNF-α mRNA expression levels. The results suggested that the suppressive activity of SDE on iNOS, COX-2 and TNF-α were mediated via transcriptional levels (Fig. 3).
The LPS-induced transcriptional control of inflammation from the activated macrophages is mainly managed by the redox-sensitive transcription factors, such as NF-κB and AP-1 [26]. We next investigated which signaling events and transcription factors were targeted by SDE using the immunoblot analyses and the reporter gene assays, to better understand the mechanisms underlying the inhibited LPS-induced cytokine production. These results suggested that the nuclear translocation pathway of NF-κB and AP-1 for the transcriptional activation of the inflammatory genes could be targeted by SDE.
The MAPK and PI3K/Akt signalling pathways play critical roles in regulating cellular proliferation, survival, and differentiation. The pathways also control the pro-inflammatory mediator synthesis and release by the activated macrophages during the inflammatory response and coordinate the induction of many genes encoding the inflammatory mediators [27,28]. Therefore, the signalling pathway is an attractive target in the anti-inflammatory drug research. The downstream signaling events that induce nuclear translocation of these transcription factors have been elucidated (Fig. 3-4). Therefore, MAPKs and PI3K/Akt pathways were selected to evaluate whether SDE was able to modulate the upstream signaling events. As the AP-1 translocation is mediated by MAPK phosphorylation, we next investigated the effects of SDE on the LPS-stimulated phosphorylation of ERK, JNK, and p38 MAPKs in the RAW 264.7 cells. Some studies have shown that p38, ERK or IκBα leads to an activation of the transcription factors (e.g., NF-κB and AP-1) and suggested that the MAPKs and PI3K/Akt signalling pathways directly affected the inflammatory protein expression [29,30]. These results suggest that SDE may block the LPS-induced expression of the pro-inflammatory cytokines by inhibiting the Akt and MAP kinase pathways.
The topical application of arachidonic acid on the mouse ears and the EtOH/HCl-induced acute gastritis models are well-known models for the induction of in vivo inflammatory symptoms [31,32]. We next explored whether SDE could ameliorate ear edema and acute gastritis in mice, induced by arachidonic acid and EtOH/HCl, respectively. The oral administration of SDE (40 mg/kg) or indomethacin (1 mg/kg) was conducted for 3 days; and arachidonic acid was applied to the ears of mice. The data suggest the SDE extract having displayed a very strong in vivo efficacy in the various inflammatory models and may be applied in the oral drugs targeting various inflammatory diseases.
In summary, we demonstratedthat SDE significantly inhibited cytokine production via down regulation of iNOS, COX-2, and TNF-α mRNA expression in the LPS-stimulated RAW 264.7 cells. Furthermore, SDE protected against the LPS-induced cell death induced by ROS via the anti-oxidative effects. In particular, the SDE extract suppressed multiple pathways of the inflammatory signaling cascades, such as Akt, p38, and ERK, which were associated with the inactivation of AP-1 and NF-κB. The in vivo tests showed that the orally administered SDE ameliorated the acute inflammatory symptoms in the EtOH/HCl-induced gastritis and arachidonic acid-induced ear edema in mice. Therefore, it is suggested that SDE may be further developed in the novel anti-inflammatory remedy research. Additional studies are currently underway to identify the specific phytochemicals responsible for the anti-inflammatory activities of SDE.
Figures and Tables
 | Fig. 1
The effects of the ethanol extract of Synurus deltoides (SDE) on in vitro inflammatory symptoms. (A) RAW264.7 cells (1×106 cells/ml) were incubated with SDE for 24 h, and the cell viability was determined using an MTT assay. (B-D) RAW264.7 cells (1×106 cells/ml) were treated with SDE in the presence or absence of lipopolysaccharide (LPS) (1 µg/ml) for 24 h. The supernatants were collected; and the nitric oxide (NO), prostaglandin E2 (PGE2), and tumour necrosis factor-α (TNF-α) concentrations were determined in the supernatants by the Griess assays and the enzyme-linked immunosorbent assays (ELISAs). (E) RAW264.7 cells (1×106 cells/ml) were treated with SDE in the presence or absence of LPS (1 µg/ml) for 24 h, and the cytoprotective effect was determined using an MTT assay. |
 | Fig. 2
Suppression of the LPS-induced reactive oxygen species (ROS) in the RAW 264.7 cells, in the presence of different concentrations of SDE. Each value is the mean ± standard deviation (n = 3). Values with the same superscript letters are not significantly different from each other at P < 0.05. |
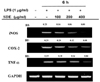 | Fig. 3
Effect of the ethanol extract of Synurus deltoides (SDE) on inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2), and tumour necrosis factor-α (TNF-α) expression in the lipopolysaccharide (LPS)-treated RAW264.7 cells. RAW264.7 cells (5×106 cells/ml) were incubated with SDE in the presence or absence of LPS (1 µg/ml) for 6 h. The levels of iNOS, COX-2, TNF-α, and GAPDH mRNA were determined by semi-quantitative polymerase chain reaction (PCR). Relative intensity (RI) was calculated by a ratio of GAPDH band intensity. The results shown are representative of three independent experiments. |
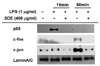 | Fig. 4
Effect of the ethanol extract of Synurus deltoides (SDE) on the translocation of the transcription factors. RAW264.7 cells (5×106 cells/ml) pre-treated with SDE for 0.5 h were stimulated with lipopolysaccharide (LPS) (1 µg/ml) for 15 and 60 min. After preparation of the nuclear fraction, levels of p65, c-Jun, c-fos, and Lammin A/C were determined by immunoblotting analysis. Results shown are representative of three independent experiments. |
 | Fig. 5
HEK 293 cells co-transfected with the plasmid constructs activating protein luciferase (NF-κB) (1 µg/ml) (A) or (AP-1-Luc) (1 µg/ml) (B) and β-gal (as a transfection control) were treated with the ethanol extract of Synurus deltoides (SDE) in the presence or absence of phorbol myristic acid (PMA) (100 nM) for 24 h. RAW 264.7 cells co-transfected with the plasmid constructs activating protein luciferase (NF-κB) (1 µg/ml) (C) or (AP-1-Luc) (1 µg/ml) (D) and β-gal (as a transfection control) were treated with the ethanol extract of Synurus deltoides (SDE) in the presence or absence of lipopolysaccharide (LPS) (1 µg/ml) for 24 h. Luciferase activity was determined by luminometry. Values with the same superscript letters are not significantly different from each other at P < 0.05. |
 | Fig. 6
Effect of the ethanol extract of Synurus deltoides (SDE) on the upstream signaling pathways for the activating protein (AP-1) and the nuclear factor-κB (NF-κB) activation. RAW264.7 cells (5×106 cells/ml) pre-treated with SDE for 30 min were stimulated with lipopolysaccharide (LPS) (1 µg/ml) for the indicated times. After immunoblotting, the levels of phospho- or total mitogen activated protein kinases (MAPKs) (ERK, p38, and JNK) (A) or IκBα and Akt (B) were identified based on their antibodies. Results are representative of three experiments. |
 | Fig. 7
Effect of the ethanol extract of Synurus deltoides (SDE) on the arachidonic acid-induced mouse ear edema. ICR mice were orally administered with SDE (40 mg/kg) or indomethacin (1 mg/kg) for 3 days. Arachidonic acid solution was topically applied (30 µl/ear) to the ear of ICR mice (n = 6). The thickness of the edema was measured with a dial thickness gauge 1 h after the arachidonic acid treatment. The ear thickness of the arachidonic acid-treatment group is represented by 100%. Values with the same superscript letters are not significantly different from each other at P < 0.05. |
 | Fig. 8
Effect of the ethanol extract of Synurus deltoides (SDE) and ranitidine on EtOH/HCl-induced gastritis. ICR mice (n = 6), orally administered with SDE or ranitidine for 3 days, were orally-treated with EtOH/HCl. After 1 h, photos of gastric lesion were taken by a camera (A), and the gastric lesions in the stomach were measured with a ruler (B). Values with the same superscript letters are not significantly different from each other at P < 0.05. |
Acknowledgement
This study was supported by the Cooperative Research Program for Agriculture Science & Technology Development (Project No. C1009413-01-01) of the Rural Development Administration and was partly supported by 2013 Research Grant from Kangwon National University (No. 120131329) Republic of Korea.
References
1. Sherwood ER, Toliver-Kinsky T. Mechanisms of the inflammatory response. Best Pract Res Clin Anaesthesiol. 2004; 18:385–405.

2. Lister MF, Sharkey J, Sawatzky DA, Hodgkiss JP, Davidson DJ, Rossi AG, Finlayson K. The role of the purinergic P2X7 receptor in inflammation. J Inflamm (Lond). 2007; 4:5.

3. Huang N, Hauck C, Yum MY, Rizshsky L, Widrlechner MP, McCoy JA, Murphy PA, Dixon PM, Nikolau BJ, Birt DF. Rosmarinic acid in Prunella vulgaris ethanol extract inhibits lipopolysaccharide-induced prostaglandin E2 and nitric oxide in RAW 264.7 mouse macrophages. J Agric Food Chem. 2009; 57:10579–10589.

4. Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 2010; 327:656–661.

5. Lee MS, Kim YJ. Signaling pathways downstream of pattern-recognition receptors and their cross talk. Annu Rev Biochem. 2007; 76:447–480.

6. Lee MS, Kwon MS, Choi JW, Shin T, No HK, Choi JS, Byun DS, Kim JI, Kim HR. Anti-inflammatory activities of an ethanol extract of Ecklonia stolonifera in lipopolysaccharide-stimulated RAW 264.7 murine macrophage cells. J Agric Food Chem. 2012; 60:9120–9129.

7. Zhong LM, Zong Y, Sun L, Guo JZ, Zhang W, He Y, Song R, Wang WM, Xiao CJ, Lu D. Resveratrol inhibits inflammatory responses via the mammalian target of rapamycin signaling pathway in cultured LPS-stimulated microglial cells. PLoS One. 2012; 7:e32195.

8. Piao W, Song C, Chen H, Diaz MA, Wahl LM, Fitzgerald KA, Li L, Medvedev AE. Endotoxin tolerance dysregulates MyD88- and Toll/IL-1R domain-containing adapter inducing IFN-beta-dependent pathways and increases expression of negative regulators of TLR signaling. J Leukoc Biol. 2009; 86:863–875.

9. Shen T, Lee J, Lee E, Kim SH, Kim TW, Cho JY. Cafestol, a coffee-specific diterpene, is a novel extracellular signal-regulated kinase inhibitor with AP-1-targeted inhibition of prostaglandin E2 production in lipopolysaccharide-activated macrophages. Biol Pharm Bull. 2010; 33:128–132.

10. Ham SS, Han HS, Choi KP, Oh DH. Antigenotoxic effects of Synurus deltoides extract on benzo[a]pyrene induced mutagenesis. J Food Sci Nutr. 1997; 2:162–166.
11. Choi YH, Son KH, Chang HW, Bae K, Kang SS, Kim HP. New anti-inflammatory formulation containing Synurus deltoides extract. Arch Pharm Res. 2005; 28:848–853.

12. Yoshitama K, Ishii K, Yasuda H. A chromatographic survey of anthocyanins in the flora of Japan, I. J Fac Sci Shinshu Univ. 1980; 15:19–26.
13. Nam JH, Choi SZ, Lee KR. Phytochemical constituents of Synurus excelsus. Korean J Pharmacogn. 2004; 35:116–121.
14. Lee HY, Min BS, Son KH, Chang HW, Kim HP, Kang SS, Bae KH. Cerebrosides and triterpenoids from the roots of Synurus deltoides. Nat Prod Sci. 2006; 12:193–196.
15. Kang TH, Pae HO, Jeong SJ, Yoo JC, Choi BM, Jun CD, Chung HT, Miyamoto T, Higuchi R, Kim YC. Scopoletin: an inducible nitric oxide synthesis inhibitory active constituent from Artemisia feddei. Planta Med. 1999; 65:400–403.

16. Park JH, Son KH, Kim SW, Chang HW, Bae K, Kang SS, Kim HP. Antiinflammatory activity of Synurus deltoides. Phytother Res. 2004; 18:930–933.
17. Wang J, Wang N, Yao X, Ishii R, Kitanaka S. Inhibitory activity of Chinese herbal medicines toward histamine release from mast cells and nitric oxide production by macrophage-like cell line, RAW 264.7. J Nat Med. 2006; 60:73–77.

18. Chon SU, Heo BG, Park YS, Cho JY, Gorinstein S. Characteristics of the leaf parts of some traditional Korean salad plants used for food. J Sci Food Agric. 2008; 88:1963–1968.

19. Hu W, Shen T, Wang MH. Cell cycle arrest and apoptosis induced by methyl 3,5-dicaffeoyl quinate in human colon cancer cells: involvement of the PI3K/Akt and MAP kinase pathways. Chem Biol Interact. 2011; 194:48–57.

20. Yu T, Ahn HM, Shen T, Yoon K, Jang HJ, Lee YJ, Yang HM, Kim JH, Kim C, Han MH, Cha SH, Kim TW, Kim SY, Lee J, Cho JY. Anti-inflammatory activity of ethanol extract derived from Phaseolus angularis beans. J Ethnopharmacol. 2011; 137:1197–1206.

21. Chung HY, Cesari M, Anton S, Marzetti E, Giovannini S, Seo AY, Carter C, Yu BP, Leeuwenburgh C. Molecular inflammation: underpinnings of aging and age-related diseases. Ageing Res Rev. 2009; 8:18–30.

22. Wu LC, Fan NC, Lin MH, Chu IR, Huang SJ, Hu CY, Han SY. Anti-inflammatory effect of spilanthol from Spilanthes acmella on murine macrophage by down-regulating LPS-induced inflammatory mediators. J Agric Food Chem. 2008; 56:2341–2349.

23. Mann PB, Kennett MJ, Harvill ET. Toll-like receptor 4 is critical to innate host defense in a murine model of bordetellosis. J Infect Dis. 2004; 189:833–836.

24. Hu W, Wang MH. Antioxidative activity and anti-inflammatory effects of diarylheptanoids isolated from Alnus hirsuta. J Wood Sci. 2011; 57:323–330.

25. Hu W, Han W, Huang C, Wang MH. Protective effect of the methanolic extract from Duchesnea indica against oxidative stress in vitro and in vivo. Environ Toxicol Pharmacol. 2011; 31:42–50.

26. Hakim A, Adcock IM, Usmani OS. Corticosteroid resistance and novel anti-inflammatory therapies in chronic obstructive pulmonary disease: current evidence and future direction. Drugs. 2012; 72:1299–1312.

27. Sun J, Ramnath RD, Tamizhselvi R, Bhatia M. Role of protein kinase C and phosphoinositide 3-kinase-Akt in substance P-induced proinflammatory pathways in mouse macrophages. FASEB J. 2009; 23:997–1010.

28. Guha M, Mackman N. The phosphatidylinositol 3-kinase-Akt pathway limits lipopolysaccharide activation of signaling pathways and expression of inflammatory mediators in human monocytic cells. J Biol Chem. 2002; 277:32124–32132.

29. Weston CR, Davis RJ. The JNK signal transduction pathway. Curr Opin Cell Biol. 2007; 19:142–149.

30. Adcock IM, Caramori G. Cross-talk between pro-inflammatory transcription factors and glucocorticoids. Immunol Cell Biol. 2001; 79:376–384.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download