This article has been corrected. See "Erratum" in Volume 7 on page 519.
Abstract
Zinc may participate in blood pressure regulation and in the pathogenesis of hypertension. The study examined the relationship between zinc status and blood pressure in obese Korean women. Forty obese women (body mass index (BMI) ≥ 25 kg/m2) aged 19-28 years participated in this study. Zinc intake was estimated from one 24 hour recall and 2-day diet records. Serum and urinary zinc concentrations were determined by atomic absorbance spectrophotometry. Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured using an automatic sphygmometer. Metabolic variables, such as waist circumference, triglyceride, high density lipoprotein (HDL) cholesterol, fasting glucose, and fasting insulin, were also measured. Dietary zinc intake of obese women was averagely 7.5 mg/day. Serum zinc and urinary zinc concentrations were 13.4 µmol/L and 378.7 µg/day, respectively. Averages of SBP and DBP were 119 mmHg and 78 mmHg. Dietary zinc intake was negatively correlated with SBP after adjusting for energy intake (P < 0.05), but serum and urinary zinc concentrations were not found to be correlated with SBP or DBP. Multivariate linear regression analysis showed that dietary zinc intake was inversely associated with SBP in obese women after adjusting for body weight, energy intake and sodium intake (P = 0.0145). The results show that dietary zinc intake may be an independent risk factor of elevated SBP in obese Korean women.
Zinc is an essential trace element in humans and animals, and is required for many physiologic functions, including growth, development, and reproduction [1]. Many enzymes contain zinc, such as alkaline phosphatase, Cu/Zn superoxide dismutase (SOD), nitric oxide synthase (NOS), neutral endopeptidase, angiotensin I-converting enzyme [2,3]. In addition, zinc has been documented to act as an antioxidant, to have membrane-stabilizing properties, to block apoptotic cell death, and to be essential for endothelial integrity [4,5].
Experimental and clinical studies suggest that zinc deficiency is involved in the pathogenesis of cardiovascular disease [6,7], and it has been reported that zinc may participate in blood pressure regulation and in the pathogenesis of hypertension [8]. However, the results of previous studies on the association between zinc status and blood pressure are controversial. For example, inverse relationships between blood pressure and dietary zinc/serum zinc concentration have been documented in hypertensive subjects [9,10], but it has also been reported that zinc status does not change blood pressure in rats [11,12] or humans [13]. However, few reports are available on the association between zinc status and blood pressure in human.
The obese appear to have lower serum zinc levels [14,15]. This low zinc status may be related to an increase of blood pressure in obese individuals. Thus, we undertook to examine the relationship between zinc status and blood pressure in obese individuals. To the best of our knowledge, no study has previously addressed the relationship between zinc status and blood pressure in obese population.
Accordingly, we examined the relationship between zinc status and blood pressure in obese Korean women and adjusted for potential confounders, such as body weight, energy intake and sodium intake.
Forty obese women (19-28 years) were recruited in the Daegu and Gyeongbuk regions. Obesity was defined as a BMI of ≥ 25 kg/m2 in accord with the International Obesity Task Force for Asian adults [16]. Participants filled out a questionnaire during a face-to-face interview. Information was collected on demographic variables, smoking, nutritional supplementation, personal medical history and family history, and medication use. Exclusion criteria included cigarette smoking, vitamin-mineral supplements and/or other nutritional supplement, use of oral contraceptives, acute or chronic disease, such as diabetes or a family history of diabetes, and participation in a weight-loss diet program. Informed written consent was obtained from each study subject prior to enrollment. The study was conducted according to the guidelines laid down in the Declaration of Helsinki and the study protocol was reviewed and approved by the Public Institutional Review Board (IRB) (PIRB12-040-02).
Usual dietary intake was estimated from 24 hour recall and 2-day diet records that included 2 weekdays and 1 weekend day. At first visit, detailed instructions for dietary records were given by trained dietitians [17]. Nutrient intakes in usual diet, including zinc, were estimated using the nutrient database developed by the Korean Nutrition Society [18,19].
Physical examinations were performed by trained research staff using standardized procedures. Height was measured by anthropometry (TKK-11252, Japan) at the time of recruitment, and body weight was measured by bioimpedance analysis (Inbody 3.0, Biospace Corp, Korea) the morning before blood collection. BMI was calculated by dividing weight (kg) by height squared (m2). Waist circumference was measured using a non-elastic tape measure at the midpoint between the lower border of the rib cage and the iliac crest. A trained research staff measured systolic blood pressure (SBP) and diastolic blood pressure (DBP) using an automatic sphygmometer (HEM-770A, Japan) after subjects had rested for 10 minutes in a sitting position immediately before blood collection. SBP and DBP were measured at phase I and V Korotkoff sound [20], respectively. Two readings of SBP and DBP were recorded and the average of two readings was used in the analysis.
Blood samples for zinc and metabolic variables analyses were collected in plastic syringes, placed on ice for a maximum of 2 hours before being centrifuged at 1,500 g for 10 min at 4℃ (Allegra 6R, Beckman Coulter, USA), and stored at -70℃ until required for analysis [21,22]. Subjects were asked to collect a 24-hour urine sample in a polyethylene container the day before the second visit for blood collection. Urine samples were mixed by shaking vigorously, weighed, and aliquots were stored at -20℃ prior to zinc analysis [21,22]. Serum and urinary zinc concentrations were determined by atomic absorbance spectrophotometry (AAS 600, Perkin Elmer, USA) [23].
Fasting glucose, triglyceride (TG), total cholesterol, and high density lipoprotein (HDL) cholesterol were measured using enzymatic methods and an automated analyzer (ADVIA 2400, Japan). Serum insulin was measured using a chemiluminescent immunoassay, and insulin resistance (IR) was determined using the homeostasis model assessment (HOMA) as fasting glucose (mmol/L) × fasting insulin (µIU/mL)/22.5. Serum leptin concentrations were measured by radioimmunoassay using a human leptin kit (Linco Research Inc., USA) and plasma adiponectin was determined by immunoassay using a human adiponectin kit (R&D Systems, USA). Serum SOD was measured using an enzyme-based method using the SOD assay kit (Cayman Chemical Company, USA).
All results are expressed as means ± standard deviations. Pearson's correlation coefficients were used to identify relationships between zinc markers and metabolic variables, and multivariate linear regression analysis was performed to examine relationship between dietary zinc intake and blood pressure in obese women. Model 1 included body weight and energy intake as covariates, which were significantly associated with blood pressure in the study subjects and potential confounders, such as body weight, energy intake and sodium intake, were adjusted for in Model 2. The sodium intake was included as a covariate because it is well-known to be associated with blood pressure. Statistical analyses were performed using SAS version 9.1 (SAS Institute Inc. NC, USA). Statistical significance was accepted for P values of < 0.05.
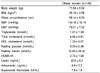
Nutrient intakes and zinc statuses in obese women are shown in Table 1. Energy intake was 1,769 kcal/day and dietary zinc intake was averagely 7.5 mg/day. More than half of obese women did not meet the RNI of zinc, which is 8 mg/day. Serum zinc and urinary zinc concentrations were 13.4 µmol/L and 378.7µg/day, respectively. Most of women were within normal range of serum zinc concentration and only three women had marginal zinc deficiency, which is below than 10.7 µmol/L.
Anthropometric measurements and metabolic variables, including blood pressure, are tabulated in Table 2. Body weight of obese women averaged 71.6 kg. Averages of SBP and DBP were 119 mmHg and 78 mmHg, which are within normal range in obese women.
Energy-adjusted correlation coefficients of relations between zinc statuses and metabolic variables are presented in Table 3. Dietary zinc intake was significantly correlated with SBP in obese subjects (P < 0.05), but serum and urinary zinc concentrations were not associated with blood pressure or other metabolic variables.
The relationship between dietary zinc intake and SBP in subjects is shown in Table 4. Dietary zinc intake was inversely associated with SBP after adjusting for body weight and energy intake (β = -2.199, P = 0.0128), and this association strengthened after adjusting for body weight, energy intake and sodium intake (β = -2.222, P = 0.0145). However, dietary zinc intake was not significantly associated with DBP, and no relation was found between blood pressure and serum or urinary zinc concentration (data not shown).
This study shows that dietary zinc intake is strongly associated with SBP in obese Korean women. Dietary zinc intake was negatively correlated with SBP after adjusting for energy intake, and multivariate regression analysis revealed an inverse association between dietary zinc and SBP after adjusting for body weight, energy intake and sodium intake. These results suggest that zinc deficiency is an independent risk factor of an elevated blood pressure in obese Korean women.
The findings of previous studies agree with our results. Tomat et al. [9] reported that moderate zinc restriction (9 mg/kg) for 60 days increased arterial blood pressure on day 30 and resulted in lower urinary excretion levels of nitrates and nitrites in three-week-old weaned male rats. Sato et al. [24] showed that spontaneously hypertensive (SH) rats fed zinc deficient diet (6.5 µg Zn/day) for 2 weeks exhibited a progressive increase in systolic blood pressure, whereas SH rats fed a standard diet (0.26 g Zn/day) did not. On the other hand, some have reported that zinc deficiency does not change blood pressure in rats or in human [11,12]. Taittonen et al. [13] found that dietary zinc was not associated with blood pressure in healthy children in a 6-year follow up study, and Sato et al. [11] found that a zinc deficient diet for 4 weeks did not influence systolic or diastolic blood pressure in normotensive rats. However, these studies used different subjects and study conditions, and thus, direct comparisons are not possible. Nevertheless, conflicting results regarding the association between zinc status and blood pressure might be due to duration of diet treatment, the degree of zinc deficiency, the period of life involved (pregnancy, fetal life, weaning, childhood, adulthood), the presence of hypertension, and the environment.
The mechanism underlying the association between zinc status and blood pressure remains unclear. However, zinc deficiency may be associated with an impaired vascular nitric oxide (NO) system due to reduced NOS activity and an increase in oxidative stress caused by superoxide. NO is an important regulator of blood flow and blood pressure in mammals, because of its vasodilatory effects. Thus, it is likely that systemic NO impairment, due to an increase in the action of superoxide, could explain increased blood pressures levels. Zinc deficiency reduces NOS activity because NOS contains zinc, and reduced NOS activity in artery walls could cause endothelial dysfunction and reduce endothelium-mediated vasodilation, and thus, contribute to the development of hypertension [25-27]. Furthermore, many enzymes that are involved in the regulation of arterial blood pressure, such as angiotensin-converting enzyme and neutral endopeptidases, contain zinc [28-30]. In addition, zinc deficiency reduces the activity of superoxide scavengers, such as, Cu/Zn SOD. Sato et al. [24] showed that the administration of NOS inhibitor increased arterial pressure, and that conversely, the administration of superoxide scavenger (Cu/Zn SOD) decreased arterial pressure in genetically hypertensive rats. Therefore, adequate zinc intake seems to be necessary to maintain endothelial cell integrity and normal blood pressure, because this zinc has antioxidant effects and membrane-stabilizing properties [4,5].
In the present study, we failed to find any significant association between serum zinc levels and blood pressure in obese women, which may have been due to zinc homeostasis. Serum zinc concentrations are maintained within a narrow range even when dietary zinc levels fluctuate [21]. In a 6-year follow up study, it was found that serum zinc levels were not correlated with blood pressure either during the year of measurement or during subsequent years in 3,596 healthy children [13], which concurs with our results. Thus, reported differences regarding relationships between markers of zinc status and blood pressure support a hypothesis of modified zinc homeostasis and of borderline zinc deficiency in tissues. Another possibility is that the women in the present study had normal blood pressures even though they are obese. This might cause no relationship between serum zinc concentration and blood pressure in obese women. Bergomi et al. [10] found no relationship between serum zinc levels and blood pressures in normotensive adults, but did find that serum zinc levels were inversely correlated with blood pressures in hypertensive subjects.
This study is limited by its cross-sectional design, which prevented the identification of a causal relationship between dietary zinc intake and the risk of an elevated blood pressure. Despite this limitation, the study involved the collection of various lifestyle factors, dietary data, and anthropometric and blood pressure measurements by trained staff using standardized protocols. Furthermore, this study is the first to examine the relationship between zinc status and blood pressure in an obese population by multivariate analysis adjusted for potential confounding factors, such as body weight, energy intake and sodium intake.
In conclusion, dietary zinc was found to be inversely associated with SBP in obese Korean women after adjusting for body weight, energy intake and sodium intake, which suggests that zinc deficiency is an independent risk factor of elevated blood pressure in this subpopulation. We recommend that large-scale clinical trials and longitudinal studies be undertaken to investigate the possibility of a causal relationship between zinc status and blood pressure in different populations.
Figures and Tables
Acknowledgment
We are grateful to study participants, the nurses, dieticians, and research members of staff that contributed to this study.
References
1. Brown KH, Wuehler SE. Zinc and Human Health: Results of Recent Trials and Implications for Program Interventions and Research. Ottawa: Micronutrient Initiative;2000.
2. Prasad AS. Zinc: an overview. Nutrition. 1995; 11:93–99.
5. Hennig B, Wang Y, Ramasamy S, McClain CJ. Zinc deficiency alters barrier function of cultured porcine endothelial cells. J Nutr. 1992; 122:1242–1247.

6. Kok FJ, Van Duijn CM, Hofman A, Van der Voet GB, De Wolff FA, Paays CH, Valkenburg HA. Serum copper and zinc and the risk of death from cancer and cardiovascular disease. Am J Epidemiol. 1988; 128:352–359.

7. Reunanen A, Knekt P, Marniemi J, Mäki J, Maatela J, Aromaa A. Serum calcium, magnesium, copper and zinc and risk of cardiovascular death. Eur J Clin Nutr. 1996; 50:431–437.
8. Tubek S. Role of zinc in regulation of arterial blood pressure and in the etiopathogenesis of arterial hypertension. Biol Trace Elem Res. 2007; 117:39–51.

9. Tomat AL, Weisstaub AR, Jauregui A, Piñeiro A, Balaszczuk AM, Costa MA, Arranz CT. Moderate zinc deficiency influences arterial blood pressure and vascular nitric oxide pathway in growing rats. Pediatr Res. 2005; 58:672–676.

10. Bergomi M, Rovesti S, Vinceti M, Vivoli R, Caselgrandi E, Vivoli G. Zinc and copper status and blood pressure. J Trace Elem Med Biol. 1997; 11:166–169.

11. Sato M, Kurihara N, Moridaira K, Sakamoto H, Tamura J, Wada O, Yanagisawa H. Dietary Zn deficiency does not influence systemic blood pressure and vascular nitric oxide signaling in normotensive rats. Biol Trace Elem Res. 2003; 91:157–172.

12. Kurihara N, Yanagisawa H, Sato M, Tien CK, Wada O. Increased renal vascular resistance in zinc-deficient rats: role of nitric oxide and superoxide. Clin Exp Pharmacol Physiol. 2002; 29:1096–1104.

13. Taittonen L, Nuutinen M, Räsänen L, Mussalo-Rauhamaa H, Turtinen J, Uhari M. Lack of association between copper, zinc, selenium and blood pressure among healthy children. J Hum Hypertens. 1997; 11:429–433.

14. Marreiro DN, Fisberg M, Cozzolino SM. Zinc nutritional status and its relationships with hyperinsulinemia in obese children and adolescents. Biol Trace Elem Res. 2004; 100:137–149.

15. Tungtrongchitr R, Pongpaew P, Phonrat B, Tungtrongchitr A, Viroonudomphol D, Vudhivai N, Schelp FP. Serum copper, zinc, ceruloplasmin and superoxide dismutase in Thai overweight and obese. J Med Assoc Thai. 2003; 86:543–551.
16. Kim J, Jo I. Relationship between body mass index and alanine aminotransferase concentration in non-diabetic Korean adults. Eur J Clin Nutr. 2010; 64:169–175.

17. Kim J, Kim HJ, Joung H, Park MK, Li S, Song Y, Franke AA, Paik HY. Overnight urinary excretion of isoflavones as an indicator for dietary isoflavone intake in Korean girls of pubertal age. Br J Nutr. 2010; 104:709–715.

18. Korean Nutrition Society. Dietary Reference Intake for Korean. Seoul: Kookjin Press;2005.
19. Kim J, Paik HY, Joung H, Woodhouse LR, King JC. Plasma zinc but not the exchangeable zinc pool size differs between young and older Korean women. Biol Trace Elem Res. 2011; 142:130–136.

20. American Society of Hypertension. Recommendations for routine blood pressure measurement by indirect cuff sphygmomanometry. American Society of Hypertension. Am J Hypertens. 1992; 5:207–209.
21. Kim J, Paik HY, Joung H, Woodhouse LR, Li S, King JC. Zinc supplementation reduces fractional zinc absorption in young and elderly Korean women. J Am Coll Nutr. 2004; 23:309–315.

22. Kim J, Paik HY, Joung H, Woodhouse LR, Li S, King JC. Effect of dietary phytate on zinc homeostasis in young and elderly Korean women. J Am Coll Nutr. 2007; 26:1–9.

23. Meret S, Henkin RI. Simultaneous direct estimation by atomic absorption spectrophotometry of copper and zinc in serum, urine, and cerebrospinal fluid. Clin Chem. 1971; 17:369–373.

24. Sato M, Yanagisawa H, Nojima Y, Tamura J, Wada O. Zn deficiency aggravates hypertension in spontaneously hypertensive rats: possible role of Cu/Zn-superoxide dismutase. Clin Exp Hypertens. 2002; 24:355–370.

25. Li H, Förstermann U. Nitric oxide in the pathogenesis of vascular disease. J Pathol. 2000; 190:244–254.

26. Moncada S. Nitric oxide in the vasculature: physiology and pathophysiology. Ann N Y Acad Sci. 1997; 811:60–67. discussion 7-9.

27. Vapaatalo H, Mervaala E, Nurminen ML. Role of endothelium and nitric oxide in experimental hypertension. Physiol Res. 2000; 49:1–10.
28. Zou MH, Shi C, Cohen RA. Oxidation of the zinc-thiolate complex and uncoupling of endothelial nitric oxide synthase by peroxynitrite. J Clin Invest. 2002; 109:817–826.

29. Dahlheim H, White CL, Rothemund J, von Lutterotti N, Jacob IC, Rosenthal J. Effect of zinc depletion on angiotensin I-converting enzyme in arterial walls and plasma of the rat. Miner Electrolyte Metab. 1989; 15:125–129.
30. Corti R, Burnett JC Jr, Rouleau JL, Ruschitzka F, Lüscher TF. Vasopeptidase inhibitors: a new therapeutic concept in cardiovascular disease? Circulation. 2001; 104:1856–1862.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download