Abstract
The purpose of this study was to investigate the effects of lotus leaf on hyperglycemia and dyslipidemia in animal model of diabetes. Inhibitory activity of ethanol extract of lotus leaf against yeast α-glucosidase was measured in vitro. The effect of lotus leaf on the postprandial increase in blood glucose levels was assessed in streptozotocin-induced diabetic rats. A starch solution (1 g/kg) with and without lotus leaf extract (500 mg/kg) was administered to the rats after an overnight fast, and postprandial plasma glucose levels were monitored. Four-week-old db/db mice were fed a basal diet or a diet containing 1% lotus leaf extract for 7 weeks after 1 week of acclimation to study the chronic effect of lotus leaf. After sacrifice, plasma glucose, insulin, triglycerides (TG), total cholesterol (CHOL), high-density lipoprotein (HDL)-CHOL, and blood glycated hemoglobin levels were measured. Lotus leaf extract inhibited α-glucosidase activity by 37.9%, which was 1.3 times stronger than inhibition by acarbose at a concentration of 0.5 mg/mL in vitro. Oral administration of lotus leaf extract significantly decreased the area under the glucose response curve by 35.1% compared with that in the control group (P < 0.01). Chronic feeding of lotus leaf extract significantly lowered plasma glucose and blood glycated hemoglobin compared with those in the control group. Lotus leaf extract significantly reduced plasma TG and total CHOL and elevated HDL-CHOL levels compared with those in the control group. Therefore, we conclude that lotus leaf is effective for controlling hyperglycemia and dyslipidemia in an animal model of diabetes mellitus.
Type 2 diabetes mellitus is characterized by abnormalities in carbohydrate, fat, and protein metabolism due to insulin resistance [1]. Cardiovascular complications are a major cause of premature mortality in patients with type 2 diabetes [2], and tight control of hyperglycemia and dyslipidemia is crucial for reducing the risk for cardiovascular diabetic complications [3,4].
α-Glucosidase inhibitors are oral hypoglycemic agents for patients with type 2 diabetes that inhibit digestion of dietary carbohydrates and thereby flatten the postprandial glucose response. Although α-glucosidase inhibitors such as acarbose and miglitol effectively alleviate both fasting and postprandial hyperglycemia [5,6], chronic use of these agents can result in gastrointestinal side effects [7]. As a result, many efforts have been made to isolate α-glucosidase inhibitors from natural products with reduced side effects, including plants. Plant leaves such as green tea [8], olive leaf [9], and guava leaf [10] have reported to show potent inhibitory activity against α-glucosidase activity.
Lotus (Nelumbo nuficera Gaertn), an aquatic perennial plant that belongs to the family Nelumbonaceae, is cultivated as a crop mainly in eastern Asia and India [11]. Its seeds, young stems, and rhizomes are consumed as food, whereas the leaves are mainly used for tea. Different parts of the lotus plant have also been used to treat diarrhea, tissue inflammation, and hemostasis in traditional medicine [12].
Lotus leaves could be helpful in the management of diabetes mellitus, as a lotus leaf extract has α-glucosidase inhibitory activity in vitro [8]. Therefore, lotus leaf is expected to be effective for preventing the rise in postprandial glucose levels, but the α-glucosidase inhibitory activity of lotus leaf had not been fully determined in vivo. Chronic consumption of lotus leaf reduces fasting blood glucose and improves blood lipid profiles in alloxan-treated mice, suggesting that it could be beneficial for managing type 1 diabetes mellitus [13]. However, the hypoglycemic and hypolipidemic effects of lotus leaf in type 2 diabetes remain unclear. This study was carried out to examine the acute effects of lotus leaf on postprandial hyperglycemia in streptozotocin (STZ)-induced diabetic rats. We also investigated the effect of chronic consumption of lotus leaf on fasting hyperglycemia and dyslipidemia in db/db mice, an animal model of type 2 diabetes that shows insulin resistance, hyperglycemia, and dyslipidemia.
Assay kits for glucose, triglycerides (TG), total cholesterol (CHOL), and high-density lipoprotein (HDL)-CHOL were obtained from Asan Co. (Seoul, Korea). An insulin assay kit and a glycated hemoglobin (HbA1C) assay kit were purchased from Linco Co. (St. Charles, MO, USA) and BioSystems (Barcelona, Spain). Yeast α-glucosidase, p-nitropheny-α-D-glucopyranoside, STZ, and all other chemical reagents were obtained from Sigma Chemical Co. (St. Louis, MO, USA).
Lotus leaves were obtained from a local market in Muan, Korea. The leaves were freeze-dried, powdered, and extracted with 10 volumes of ethanol for 12 h twice at room temperature, and the solvent was removed by rotary evaporation [14]. The extraction yield was 12.3%. The dry extract was redissolved in dimethyl sulfoxide at a concentration of 5 mg/mL to be used as a test material for the in vitro study. The solvent was removed by rotary evaporation.
Yeast α-glucosidase inhibitory activity was measured using a microplate reader (model 550; Bio-Rad, Hercules, CA, USA) according to the method described by Watanabe et al. [15]. Yeast α-glucosidase (0.7 U) dissolved in 100 mM phosphate buffer (pH 7.0) containing 2 g/L bovine serum albumin and 0.2 g/L NaN3 and 5 mM p-nitrophenyl-α-D-glucopyranoside in the same buffer (pH 7.0) was used as enzyme and substrate solutions, respectively. The final concentration of the lotus leaf extract and acarbose, a positive control, was 0.5 mg/mL. Measurements were performed in triplicate.
Male Sprague-Dawley rats (weight 220-250 g; Bio Genomics, Inc., Seoul, Korea) were fed a commercial chow (Samyang Co., Seoul, Korea) ad libitum for 2 weeks after arrival. The animals were maintained under standard laboratory conditions of 24 ± 5℃ and 55 ± 5% relative humidity with a 12-h light:12-h dark cycle. STZ (60 mg/kg) in citrate buffer (pH 4.5) was injected intraperitoneally into the animals to induce diabetes [16]. Blood samples were withdrawn from the tail tip after 1 week, and blood glucose concentrations were measured using a glucometer (Glucotrend; Roche Diagnostics, West Sussex, UK). Animals with fasting blood glucose levels > 200 mg/dL (n = 16) were considered diabetic and randomly divided into three groups. After an overnight fast, the animals were administered soluble starch (1 g/kg) alone (control group), or starch with an ethanol extract of lotus leaf (500 mg/kg) by gastric intubation [14]. Feed was withheld during the test. Blood samples were collected from the tail vein after 30, 60, 120, 180, and 240 min and centrifuged at 1,000 g for 15 min. Plasma glucose was measured by enzymatic method using a commercial glucose assay kit [17]. Plasma glucose levels are expressed as increases from the baseline, and increases in the areas under the response curves (AUC) were calculated using the trapezoidal rule.
Five-week-old male C57BL/KsJ-db/db mice (n = 16) were purchased from SLC Japan (Shizuoka, Japan). The animals had free access to commercial food during a 1-week acclimation period and were then randomly divided into a control and the lotus-leaf groups. The mice in the control group were offered an AIN-93G diet composed of 39.8% cornstarch, 20% casein, 13.2% dextrinized cornstarch, 10% sucrose, 7% soybean oil, 5% Alphacel, 3.5% mineral mixture, 1% vitamin mixture, 0.3% L-cysteine, 0.25% choline bitartrate, and 0.0014% tert-butyl hydroquinone [18], whereas the lotus leaf group was fed the same diet supplemented with 1% (w/w, final concentration) lotus leaf extract in place of cornstarch ad libitum for 7 weeks. After an overnight fast, the mice were sacrificed by heart puncture. Plasma glucose [17], TG [19], total CHOL [20], and HDL-CHOL levels [21] were measured by enzymatic methods using commercial assay kits. Blood HbA1C [22] and plasma insulin levels [23] were measured using a chromatographic assay and radioimmunoassay kits, respectively. The homeostasis model assessment for insulin resistance (HOMA-IR) was calculated by dividing the product of insulin (µU/mL) and glucose (mmol/L) by 22.5 [24].
All animal experiments were performed according to the guidelines of animal experimentation approved by the Animal Resource Center at Inje University, Korea.
The in vitro inhibitory activity of the lotus leaf ethanol extract against yeast α-glucosidase is shown in Table 1. The lotus leaf extract inhibited yeast α-glucosidase activity by 37.9% at a concentration of 0.5 mg/mL, whereas acarbose showed a 29.6% inhibition.
The effect of lotus leaf on the postprandial rise in blood glucose in STZ-induced diabetic rats was determined by carbohydrate load testing. Oral administration of lotus leaf extract (500 mg/kg) significantly decreased the increase in plasma glucose levels at 30 (P < 0.05), 60 (P < 0.01), and 120 min (P < 0.05) after starch loading (1 g/kg) (Fig. 1). Consumption of the lotus leaf extract in the rats decreased the AUCs for the postprandial glucose responses by 35.1%, compared with those in the control group (P < 0.01; Table 2).
Body weight, food intake, and feed efficiency ratio of db/db mice were not significantly influenced by consuming the lotus leaf extract (1% of diet; Table 3). Plasma glucose levels were significantly lower in the lotus leaf group (427.6 ± 18.3 mg/dL) than in the control group (496.0 ± 21.3 mg/dL; P < 0.05; Fig. 2). Plasma insulin levels of the lotus leaf group (63.8 ± 2.8 µU/mL) were not significantly different from that of the control group (65.3 ± 2.5 µU/mL), whereas consuming the lotus leaf extract significantly reduced the HOMA-IR value by 15.6% compared with that in the control group (P < 0.01). HbA1C levels in the lotus leaf group (7.5 ± 0.3%) were lower than those in the control group (8.4 ± 0.3%; P < 0.05).
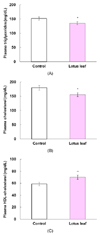
Consumption of the lotus leaf extract significantly lowered plasma TG (135.4 ± 5.5 mg/dL) and total CHOL levels (155.5 ± 7.0 mg/dL) compared with those in the control group (152.0 ± 5.7 and 180.0 ± 8.2 mg/dL, respectively; P < 0.05). Plasma HDL-CHOL levels were significantly elevated in the lotus leaf group (70.2 ± 3.6 mg/dL) compared with those in the control group (58.8 ± 2.9 mg/dL; P < 0.01; Fig. 3).
We determined the α-glucosidase inhibitory activity of a lotus leaf extract in vitro and in vivo to evaluate the potential of the lotus leaf extract as a hypoglycemic agent for diabetes mellitus. Yeast α-glucosidase inhibitory activity of the lotus leaf extract was 1.3 times stronger than that of acarbose at a concentration of 0.5 mg/mL in vitro (Table 1). Lin et al. [25] isolated catechin and quercetin from lotus leaf as its active constituents; catechin [26] and quercetin [27,28] have strong inhibitory activity against α-glucosidase in vitro and in vivo. Therefore, the α-glucosidase inhibitory activity of lotus leaf could be due to catechin and quercetin content.
The inhibitory activity of the lotus leaf extract against α-glucosidase was further determined in STZ-induced diabetic rats to verify its effect in vivo. A single oral dose of lotus leaf extract (500 mg/kg) was effective in flattening the postprandial rise in plasma glucose levels (Fig. 1) and reducing the AUC of the glucose response curve in the animals (Table 2). Control of both fasting and postprandial hyperglycemia is crucial for maintaining tight control of blood glucose levels in patients with diabetes [29]. Increasing evidence from epidemiological and interventional studies suggests that postprandial hyperglycemia might be a more important marker of risk for cardiovascular diabetic complications than fasting hyperglycemia [30]. Thus, alleviating postprandial hyperglycemia by administering lotus leaf extract could be helpful in the management of diabetes.
The hypoglycemic and hypolipidemic effect of chronic consumption of lotus leaf was determined in db/db mice. Lotus leaf extract consumed at 1% of the total diet decreased fasting plasma glucose and blood HbA1c by 13.8% and 10.5%, respectively, without influencing insulin levels in db/db mice (Fig. 2). The lotus leaf extract reduced the HOMA-IR index, suggesting improved insulin resistance. Impaired postprandial glucose elevations by acarbose, an α-glucosidase inhibitor, has been demonstrated to alleviate glucose toxicity, resulting in overall blood glucose control [31]. In addition, consuming a lotus leaf extract for 2 weeks improved insulin sensitivity in high-fat diet-fed mice determined by the insulin tolerance test [32]. Lotus leaf extract may be able to improve fasting hyperglycemia by reducing glucose toxicity and increasing insulin sensitivity. HbA1c is considered to be the best marker for long-term blood glycemic control [33] and is strongly associated with the incidence of diabetic complications [34,35]. Thus, reduced blood glucose and HbA1c in response to lotus leaf treatment could contribute to a lower risk of diabetic complications.
Insulin resistance has been reported to contribute to development of dyslipidemia [36-38]. Insulin resistance elevates free fatty acid flux, increasing hepatic production of TG and very-low-density lipoprotein (VLDL), which is converted to TG-rich remnants [36]. VLDL remnants interfere with the clearance of chylomicron remnants, causing hypertriglyceridemia [37]. Increased exchange between the cholesteryl esters of HDL and TG of TG-rich lipoproteins lowers blood HDL-CHOL [38]. Thus, insulin resistance could induce hypertriglyceridemia and reduction in HDL-CHOL in db/db mice. However, the results of this study showed that the lotus leaf extract effectively alleviated hypertriglyceridemia and hypercholesterolemia and elevated HDL-CHOL levels in db/db mice (Fig. 3). Therefore, improved insulin sensitivity due to administration of a lotus leaf extract could contribute to controlling dyslipidemia, which is important in reducing the risk of micro and macrovascular complications in patients with diabetes [39].
Hypolipidemic effect of lotus leaf could have been partly mediated by catechin and quercetin in this study. Catechin ameliorates plasma lipid profiles by reducing lipogenic gene expression such as sterol regulatory element-binding protein-1c (SREBP-1c) and fatty acid synthase (FAS) and inducing lipolytic gene expression such as hormone-sensitive lipase (HSL) and adipose triglyceride lipase (ATGL) in high-fat diet-induced obese mice [40]. Quercetin decreases TG synthesis by reducing SREBP-1c expression in the liver of mice fed a western diet [41]. The lotus leaf extract could modulate gene expression of SREBP-1c, FAS, HSL, or ATGL. Further study is necessary to identify the active components that mediate the hypoglycemic and hypolipidemic effects of lotus leaf and to understand the underlying mechanisms of action.
In conclusion, lotus leaf was effective in controlling postprandial hyperglycemia in STZ-induced diabetic rats and fasting hyperglycemia in db/db mice. Lotus leaf also alleviated hypertriglyceridemia and hypercholesterolemia and increased HDL-CHOL in db/db mice. These results suggest that lotus leaf could play a beneficial role in management of hyperglycemia and dyslipidemia in animal model of diabetes mellitus.
Figures and Tables
 | Fig. 1
Increase in blood glucose after administration of lotus leaf extract in STZ-induced diabetic rats. Control group (●): Starch (1 g/kg) was administered orally to streptozotocin-induced diabetic rats after an overnight fast. Lotus leaf group (■): Starch (1 g/kg) plus ethanol extract of lotus leaf (500 mg/kg) was administered orally to the rats after an overnight fast. Values represent means ± SE (n = 8). *Significantly different at P < 0.05, **Significantly different at P < 0.01, ns; not significant. |
 | Fig. 2
Hypoglycemic effects of lotus leaf extract in db/db mice. (A) Blood glycated hemoglobin (HbA1C), (B) Plasma glucose, (C) Plasma insulin and (D) HOMA-IR. The control group was fed a standard AIN-93G diet, whereas the treatment group was fed a diet containing 1% ethanol extract of lotus leaf ad libitum for 7 weeks. Values represent means ± SE (n = 8). *Significantly different at P < 0.05, ns; not significant. |
 | Fig. 3
Hypolipidemic effects of lotus leaf extract in db/db mice. (A) Plasma triglycerides, (B) Plasma total cholesterol and (C) Plasma HDL-cholesterol. The control group was fed a standard AIN-93G diet, whereas the treatment group was fed a diet containing 1% ethanol extract of lotus leaf ad libitum for 7 weeks. Values represent means ± SE (n = 8). *Significantly different at P < 0.05, **Significantly different at P < 0.01. |
Table 2
Area under the glucose response curve of STZ-induced diabetic rats

Control group: Starch (1 g/kg) was administered orally to streptozotocin-induced diabetic rats after an overnight fast. Lotus leaf group: Starch (1 g/kg) plus ethanol extract of the lotus leaf (500 mg/kg) was administered orally to the rats after an overnight fast. Values represent means ± SE (n = 8). **Significantly different at P < 0.01.
References
1. King H, Aubert RE, Herman WH. Global burden of diabetes, 1995-2025: prevalence, numerical estimates, and projections. Diabetes Care. 1998; 21:1414–1431.

3. American Diabetes Association. Summary of revisions for the 2008 clinical practice recommendations. Diabetes Care. 2008; 31:S3–S4.
4. American Diabetes Association. Management of dyslipidemia in adults with diabetes. Diabetes Care. 1998; 21:179–182.
5. Standl E, Baumgartl HJ, Füchtenbusch M, Stemplinger J. Effect of acarbose on additional insulin therapy in type 2 diabetic patients with late failure of sulphonylurea therapy. Diabetes Obes Metab. 1999; 1:215–220.

6. Sels JP, Huijberts MS, Wolffenbuttel BH. Miglitol, a new alphaglucosidase inhibitor. Expert Opin Pharmacother. 1999; 1:149–156.
7. Hanefeld M. The role of acarbose in the treatment of noninsulin-dependent diabetes mellitus. J Diabetes Complications. 1998; 12:228–237.

8. Mai TT, Thu NN, Tien PG, Van Chuyen N. Alpha-glucosidase inhibitory and antioxidant activities of Vietnamese edible plants and their relationships with polyphenol contents. J Nutr Sci Vitaminol (Tokyo). 2007; 53:267–276.

9. Gonzalez M, Zarzuelo A, Gamez MJ, Utrilla MP, Jimenez J, Osuna I. Hypoglycemic activity of olive leaf. Planta Med. 1992; 58:513–515.

10. Wang H, Du YJ, Song HC. α-Glucosidase and α-amylase inhibitory activities of guava leaves. Food Chem. 2010; 123:6–13.

11. Sridhar KR, Bhat R. Lotus - a potential nutraceutical source. J Agric Technol. 2007; 3:143–155.
12. Mukherjee PK, Mukherjee D, Maji AK, Rai S, Heinrich M. The sacred lotus (Nelumbo nucifera) - phytochemical and therapeutic profile. J Pharm Pharmacol. 2009; 61:407–422.

13. Zhou T, Luo D, Li XY, Luo Y. Hypoglycemic and hypolipidemic effects of flavonoids from lotus (Nelumbo nuficera Gaertn) leaf in diabetic mice. J Med Plant Res. 2009; 3:290–293.
14. Lee SK, Hwang JY, Song JH, Jo JR, Kim MJ, Kim ME, Kim JI. Inhibitory activity of Euonymus alatus against alphaglucosidase in vitro and in vivo. Nutr Res Pract. 2007; 1:184–188.

15. Watanabe J, Kawabata J, Kurihara H, Niki R. Isolation and identification of α-glucosidase inhibitors from tochu-cha (Eucommia ulmoides). Biosci Biotechnol Biochem. 1997; 61:177–178.

16. Akbarzadeh A, Norouzian D, Mehrabi MR, Jamshidi S, Farhangi A, Verdi AA, Mofidian SM, Rad BL. Induction of diabetes by streptozotocin in rats. Indian J Clin Biochem. 2007; 22:60–64.

17. Raabo E, Terkildsen TC. On the enzymatic determination of blood glucose. Scand J Clin Lab Invest. 1960; 12:402–407.

18. Reeves PG, Nielsen FH, Fahey GC Jr. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993; 123:1939–1951.

19. Grossman SH, Mollo E, Ertingshausen G. Simplified, totally enzymatic method for determination of serum triglycerides with a centrifugal analyzer. Clin Chem. 1976; 22:1310–1313.

20. Kattermann R, Jaworek D, Möller G, Assmann G, Björkhem I, Svensson L, Borner K, Boerma G, Leijnse B, Desager JP, Harwengt C, Kupke I, Trinder P. Multicentre study of a new enzymatic method of cholesterol determination. J Clin Chem Clin Biochem. 1984; 22:245–251.

21. Wadehra NR. A colorimetric method for the estimation of cholesterol from high density lipoprotein and its subclasses. Indian J Clin Biochem. 1990; 5:131–134.

22. Schifreen RS, Hickingbotham JM, Bowers GN Jr. Accuracy, precision, and stability in measurement of hemoglobin A1c by "high-performance" cation-exchange chromatography. Clin Chem. 1980; 26:466–472.

23. Morgan CR, Lazarow A. Immunoassay of insulin: two antibody system, plasma insulin levels in normal, subdiabetic and diabetic rats. Diabetes. 1963; 12:115–126.

24. Haffner SM, Miettinen H, Stern MP. The homeostasis model in the San Antonio Heart Study. Diabetes Care. 1997; 20:1087–1092.

25. Lin HY, Kuo YH, Lin YL, Chiang W. Antioxidative effect and active components from leaves of Lotus (Nelumbo nucifera). J Agric Food Chem. 2009; 57:6623–6629.

26. Matsui T, Tanaka T, Tamura S, Toshima A, Tamaya K, Miyata Y, Tanaka K, Matsumoto K. alpha-Glucosidase inhibitory profile of catechins and theaflavins. J Agric Food Chem. 2007; 55:99–105.
27. Jo SH, Ka EH, Lee HS, Apostolidis E, Jang HD, Kwon YI. Comparison of antioxidant potential and rat intestinal α-glucosidases inhibitory activities of quercetin, rutin, and isoquercetin. Int J Appl Res Nat Prod. 2009; 2:52–60.
28. Kim JH, Kang MJ, Choi HN, Jeong SM, Lee YM, Kim JI. Quercetin attenuates fasting and postprandial hyperglycemia in animal models of diabetes mellitus. Nutr Res Pract. 2011; 5:107–111.

29. Abrahamson MJ. Optimal glycemic control in type 2 diabetes mellitus: fasting and postprandial glucose in context. Arch Intern Med. 2004; 164:486–491.

30. Wajchenberg BL. Postprandial glycemia and cardiovascular disease in diabetes mellitus. Arq Bras Endocrinol Metabol. 2007; 51:212–221.

31. Lebovitz HE. α-Glucosidase inhibitors as agents in the treatment of diabetes. Diabetes Rev. 1998; 6:132–145.
32. Huang CF, Chen YW, Yang CY, Lin HY, Way TD, Chiang W, Liu SH. Extract of lotus leaf (Nelumbo nucifera) and its active constituent catechin with insulin secretagogue activity. J Agric Food Chem. 2011; 59:1087–1094.

33. Sacks DB, Bruns DE, Goldstein DE, Maclaren NK, McDonald JM, Parrott M. Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Clin Chem. 2002; 48:436–472.

34. Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM. Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002; 346:393–403.

35. O'Keefe JH Jr, Miles JM, Harris WH, Moe RM, McCallister BD. Improving the adverse cardiovascular prognosis of type 2 diabetes. Mayo Clin Proc. 1999; 74:171–180.
36. Taskinen MR. Diabetic dyslipidaemia: from basic research to clinical practice. Diabetologia. 2003; 46:733–749.

37. Brown WV, Clark L, Falko JM, Guyton JR, Rees TJ, Schonfeld G, Lopes-Virella MF. Optimal management of lipids in diabetes and metabolic syndrome. J Clin Lipidol. 2008; 2:335–342.

38. Guérin M, Le Goff W, Lassel TS, Van Tol A, Steiner G, Chapman MJ. Atherogenic role of elevated CE transfer from HDL to VLDL(1) and dense LDL in type 2 diabetes : impact of the degree of triglyceridemia. Arterioscler Thromb Vasc Biol. 2001; 21:282–288.
39. Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, Hadden D, Turner RC, Holman RR. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000; 321:405–412.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download