Abstract
3T3-L1 preadipocyte were differentiated to adipocytes, and then treated with 0, 10, 20, and 40 µg/mL of peanut sprout ethanol extract (PSEE). The main component of PSEE is resveratrol which contained 5.55 mg/mL of resveratrol. The MTT assay, Oil-Red O staining, glycerol-3-phosphate dehydrogenase (GPDH) activity, and the triglyceride concentration were determined in 3T3-L1 cells. MMP-2 and MMP-9 activities as well as mRNA expressions of C/EBP β and C/EBP α were also investigated. As the concentration of PSEE in adipocytes increased, the cell proliferation was decreased in a dose-dependent manner from 4 days of incubation (P < 0.05). The GDPH activity (P < 0.05) and the triglyceride concentration (P < 0.05) were decreased as the PSEE treatment concentration increased. The mRNA expression of C/EBPβ in 3T3-L1 cells was significantly low in groups of PSEE-treated, compared with control group (P < 0.05). The MMP-9 (P < 0.05) and MMP-2 (P < 0.05) activities were decreased in a dose-dependent manner as the PSEE concentration increased from 20 µg/mL. In conclusion, it was found that PSEE has an effect on restricting proliferation and differentiation of adipocytes.
Obesity refers to not only the gaining of weight, but accumulation of adipose tissue in body that poses a significant health problem. Adipocyte hypertrophy stimulates lipogenesis, increasing the overall storage of lipids within adipocytes and resulting in increased mass of adipose tissues [1]. Adipocyte hyperplasia due to preadipocyte proliferation and differentiation also contributes to adipose mass expansion [2]. Angiogenesis leads to cell division and division of extracellular matrix (ECM) by releasing proteases. The endothelial cells then move and combine with each other, forming new vessels which connect with existing capillaries and accelerating formation and growth of new adipose tissues [3]. Matrix metalloproteinases (MMPs) are one of the proteases which breaks down ECM during angiogenesis [4]. Preadipocyte treatment with MMP inhibitors, markedly decreased adipocyte differentiation [4].
Resveratrol possesses numerous health benefits such as anti-inflammatory functions [5], antioxidant [6], restriction of platelet aggression [7], decrease in cholesterol level, and prolonged life [8]. It also induces oxidation of fatty acids and restricts lipiogenesis [9], while stimulating lipolysis [10]. Animal experiment has shown that adding resveratrol to a high calorie diet reduced the rate of weight gain, as compared to a high calorie diet only [11].
Wine has generally been known as a good source of resveratrol, and it contains between 0.2 and 5.8 mg/L, depending on the grape variety and extraction method [12]. Peanuts are also a good source of natural resveratrol, and the resveratrol is found to be higher in peanut sprouts than in peanuts [13]. Peanuts contain 1.18-1.52 µg/g of resveratrol, while peanut sprouts contain resveratrol ranging 12.0-47.1 µg/g, depending on the germination period and geographic origin [13]. Wang et al. [13] reported that resveratrol content in peanut sprouts significantly increased from 4.5 µg/g at 0 day of germination, to 25.7 µg/g at 9 days of germination. In addition to increased resveratrol contents, total crude protein and amino acids of peanut sprouts also increased during germination, while total crude fat contents of peanut sprouts decreased. However, the levels of some unsaturated fatty acids in peanut sprouts, such as oleic and linoleic acid, were found to be high, compared with peanut [14].
Studies related with peanut sprouts mostly focused on the nutritional analysis, antioxidant activity or toxicity of peanut sprouts [13-16], and research on the effects of peanut sprouts extracts on adipocytes proliferation and differentiation have not been conducted. The result of previous studies regarding resveratrol and anti-obesity effect leads to the hypothesis that peanut sprout, which is good source of resveratrol, may also effective in suppressing adipocyte differentiation and proliferation. Taken together, we expect that peanut sprout may has a potential food in the treatment or prevention of obesity as they may act as a regulator of lipid metabolism in fat tissues. Thus this research tried to find the effects of peanut sprout ethanol extract (PSEE) on the proliferation and differentiation of adipocytes, and the activity of MMPs.
The peanut sprout ethanol extract (PSEE) used in the experiment, which contained 5.55 mg/mL of resveratrol, was manufactured and distributed by Chonnam National University, Korea.
The PSEE was dissolved in dimethyl sulphoxide (DMSO) (Sigma, St. Louis, MO, USA) to make a 40 mmol/L stock. This stock solution was stored in -20℃ and diluted in Dulbecco's modified Eagle's medium (DMEM) (WelGENE, Daegu, Korea) for each experiment. All other reagents, unless otherwise indicated, were purchased from Sigma (Sigma, St. Louis, MO, USA).
A mouse fibroblast 3T3-L1 preadipocyte were purchased from the American Type Culture Collection (Rockville, MD, USA). The cells were cultured into a regular medium (RM) containing Dulbecco's modified Eagle's medium (DMEM) (WelGENE, Daegu, Korea), 10% bovine serum albumin (BSA) (WelGENE, Daegu, Korea), 100 units/mL penicillin, and 100 µg/mL streptomycin (WelGENE, Daegu, Korea), at 37℃ in 5% CO2 incubator [17]. The media was exchanged every 2 days until it became post-confluent. When the cell was 70-80% confluent, it was rinsed twice with phosphate buffered saline (PBS). The cell was separated with trypsin-EDTA (WelGENE, Daegu, Korea), and then sub-incubated with the medium.
To differentiate 3T3-L1 preadipocytes to adipocytes, cells were incubated with differentiation medium containing 10% fetal bovine serum (FBS, WelGENE, Daegu, Korea), 0.5 mmol/L isobuthylmethylxanthine (IBMX), 10 µg/mL insulin, 1 µmol/L dexamethasone (Dex), 100 units/mL penicillin, and 100 µg/mL streptomycin for 48 h [18]. Two days after differentiation, adipocytes were incubated with post differentiation media (PM), which consisted of DMEM containing 10% FBS, 10 µg/mL insulin, 100 units/mL penicillin, and 100 µg/mL streptomycin.
To find the effects of PSEE on adipocyte proliferation, an MTT assay was conducted [19]. 3T3-L1 preadipocytes were plated in 24 well plates (1.5 × 104 cells/mL). After 2 days, cells were treated with 0, 10, 20, or 40 µg/mL (0, 40, 80, or 160 µmol/L of resveratrol) of PSEE for 48 h at 37℃. MTT [1 mg/mL 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay was conducted at 0, 2, 4, or 6 days. The plate treated with MTT was then incubated 3 h at 37℃, 5% CO2 incubator. After incubation, the MTT solution was removed, and 0.5 mL isopropyl alcohol was added; and then the plates were read at 490 nm using spectrophotometer (Tecan Austria GmbH, USA). The experiment was conducted 3 times independently, and the cell viability was expressed as percentage over control.
The intercellular lipid accumulation within adipocytes was determined by Oil-Red O solution [20]. The cells were incubated in differentiation medium containing various concentrations of PSEE for 6 days of incubation. After incubation, cells which were treated with PSEE were rinsed twice with 100 µL PBS and fixed with 50 µL Oil-Red O solution for 15 min at room temperature. Excess Oil-Red O dye was washed with distilled water, and 50 µL of dye extraction solution for the stained oil droplets in cells was added to each well, shaken 30 min at room temperature, and the absorbance was measured at 520 nm using a spectrophotometer (Tecan Austria GmbH, USA). The difference in absorbance between samples with and without dye solution was calculated.
A triglyceride assay kit (Wako chemicals GmbH, Neuss, Germany) was used for determining triglyceride concentration within cells [21]. After cells were treated with PSEE, the medium was replaced with lysis buffer (137 mmol/L NaCl, 20 mmol/L Tris-Cl, 1% triton X-100, 10% glycerol, 1 mmol/L sodium orthovanadate, 1 mmol/L PMSF, 20 µg/mL aprotinin, 10 µg/mL antipain, 10 µg/mL leupeptin, and 80 µg/mL benzamidine HCl) to lysis the cell. The cell lysate was homogenized by supersonic waves on ice, and centrifuged at 4℃ and 12,800 rpm. Then chloroform was added into cell lysate and once again centrifuged at 4℃ and 12,800 rpm for 5 min. Chloroform layer was carefully collected and dried. The pallet was melted with 1% Triton X-100, added with color reagent 300 µL, and then incubated for 5 min at 37℃ in a 5% CO2 incubator. The incubation absorbance was the measured with spectrophotometer (Tecan Austria GmbH, USA) at 600 nm.
GPDH activity was measured using commercial GPDH assay kit (Takara Bio Inc. Otsu, Shiga, Japan) [22]. The cells incubated with post differentiation medium containing PSEE for 2 days were washed twice with PBS and dissolved in enzyme extraction solution. Then the cell lysate was mixed well, and the change of absorbance was measured every minute for 10 min in spectrophotometer (Tecan Austria GmbH, USA) at 340 nm, 30℃. The GPDH activity was expressed as the amount of enzyme needed for 1 µmol/L of substrate per min at 30℃, then converted to mg protein/mL. The amount of protein was calculated with a Bio-Rad protein assay kit (BioRad, Richmond, CA, USA).
After 3 days of incubations of the cells treated PSEE with post differentiation medium, C/EBP β and C/EBP α mRNA expressions were investigated [23-27]. Total RNA was isolated using TRI-reagent, and cDNA was synthesized using 3 µg of total RNA with SuperScript II reverse transcriptase. Sybr green Master Mix (AB, Applied Biosystems, CA, USA), primers for C/EBP β (upstream primer, 5'-TTACAACAGGCCAGGTTTCC-3'; downstream primer, 5'-GGCTGGCGACATACAGTACA-3') or C/EBP α (upstream primer, 5'-GTTTCGGGACTTGATGCAATC-3'; downstream primer, 5'-AACAACCCCGCAGGAACAT-3'), and nuclease-free water were added into 1 µg of cDNA, to fix the final volume of 20 µL. Real Time PCR (AB, Applied Biosystems, CA, USA) was annealed at 95℃ with gradual increase of 0.3℃ per s with 40 cycles: 95℃ for 10 min, at the first stage: 95℃ for 5 s, and 60℃ for 1 min at the second stage,: 95℃ for 15 s, and 60℃ for 1 min at the third stage; and finally reaching 95℃. The mRNA expression of each group was normalized using β-actin as internal control and analyzed with RQ value.
After treating the cells with PSEE; the media were collected and centrifuged for 10 min at 3,000 rpm. The supernatant was separated by polyacryamide gel ectrophoresis containing 1% gelatin. It was shaken 2 times for 30 min in the gel renaturing buffer (Triton X-100 2.5% in water) (v/v); and shaken again for 30 min in a developing buffer (0.5 mmol/L Tris, Tris-HCl, NaCl, CaCl2, Brij) [28]. The cell lysate was dyed with 0.25% commassie blue for 30 min and then destained by shaking destain solution (methanol: acetic acid: distilled water, 50:10:40) (v/v) for 10 min. After washing the cells with distilled water, the dyed bands of the activated parts were scanned.
The experiments of this research were conducted 3 times independently, and the results were analyzed with an SAS Program. The value was expressed with the mean and standard error. The treatment differences were determined by ANOVA analysis, and Duncan's multiple range test was run at α = 0.05 to check for significance.
Within 2 days of treatments, no significant effect on cell proliferation was seen (Fig. 1). However, after 4 days of treatment, PSEE treatment of 40 µg/mL inhibited the proliferation of 3T3-L1 cells compared to the control (no PSEE treatment) (P < 0.05). At 6 days after PSEE treatment, cell proliferation significantly decreased by 79.6% at 20 µg/mL of PSEE (P < 0.05) and 59.7% at 40 µg/mL of PSEE (P < 0.05) compared to the control.
When the lipid accumulation during adipocyte differentiation was measured using Oil-Red O staining, the staining level was significantly attenuated with various PSEE treatment after 4 days of incubation (Fig. 2). In days 4, lipid accumulation in 3T3-L1 cells was significantly inhibited by 96.5% at PSEE treatments of 20 µg/mL (P < 0.05), and 93.7% at PSEE treatments of 40 µg/mL (P < 0.05), respectively, compared to the control. In days 6, only significant difference between PSEE treatment of 40 µg/mL and the control was shown (P < 0.05).
When 3T3-L1 cells were treated with PSEE at 10, 20, or 40 µg/mL, there was significant difference in TG concentration only between 40 µg/mL of PSEE treatment and the control (P < 0.05) (Fig. 3).
PSEE treatment of 40 µg/mL in 3T3-L1 cells showed the highest inhibition of GPDH activity (Fig. 4). GPDH activity were decreased by 98.9%, 76.8% (P < 0.05), and 62.7% (P < 0.05) according to PSEE treatments with 10, 20, and 40 µg/mL, respectively, compared to the control.
The mRNA expressions of C/EBPβ in 3T3-L1 cells treated with PSEE were significantly low compared with the control (P < 0.05) (Fig. 5). Meanwhile, the mRNA expressions of C/EBPα did not differ between the control and 10, 20, or 40 µg/mL of PSEE treatments.
Compared to the control, MMP-2 activity significantly decreased to 71% at 20 µg/mL of PSEE (P < 0.05) and 69% at 40 µg/mL of PSEE (P < 0.05) (Fig. 6A). The activity of MMP-9 decreased to 91%, 75%, and 58% by the increased concentration of PSEE at 10, 20, or 40 µg/mL, with significant difference (P < 0.05) (Fig. 6B).
Resveratrol is known to restrict adipocyte differentiation and proliferation, and various studies are being held on the effects of resveratrol on controlling the body fat [8-11]. Recently, the Rural Development Administration (RDA) of Korea reported that the germination of peanuts increased the amount of resveratrol more than 90 times as the amount of resveratrol in peanuts [29]. Shin's study found that various sprouted vegetables extracted with ethanol restricted differentiation of 3T3-L1 preadipocytes [30]. As such, the purpose of this study was to find the physiological effects of peanut sprout on adipocytes.
To find the suppressive effects of PSEE on adipocyte differentiation, Oil-Red O staining, GPDH activity, and TG accumulation were determined. The Oil-Red O solution used in the experiment only dyes TGs and cholesterol ester, and by treating it with peanut sprout, there was a dose-dependent decrease of adipocyte differentiation, suggesting that peanut sprouts cause a greater reduction of lipid accumulation.This study also showed that peanut sprout ethanol extract (PSEE) inhibited GPDH activity in a does independent manner. Since GPDH is a key enzyme of fatty acid and triacylglycerol synthesis in adipocytes, which increases during differentiation of preadipocytes into adipocytes [31], this study suggest that peanut sprouts cause a greater reduction of lipid accumulation in mature adipocytes by inhibiting GDPH activity.
Rayalam et al. [32] found that after differentiating 3T3-L1 adipocyte into mature adipocytes and treating it with a resveratrol concentration of 0, 12.5, 25, or 50 µmol/L, there was a significant decrease in cell proliferation at 25 and 50 µmol/L, which is a similar result as our study. Zhang et al. [33] reported that the resveratrol content of resveratrol-amplified grape skin extracts was higher in the 80% ethanol extract (3.06 mg/g) than 50% ethanol extract (2.57 mg/g), and adipocytes treated with the 80% ethanol extracts showed reduced adipocyte proliferation and differentiation compared with the differentiated media cells.
We also previously reported that after six days of 10, 20, and 40 µmol/L resveratrol treatment, lipid accumulation and GPDH activity in adipocyte was significantly inhibited as a dose-dependent response (P < 0.05) [34]. Park et al. [35]'s findings were also consistent with our study in showing that resveratrol treatment of 3T3-L1 adipocytes had a significant apoptosis and inhibition of GPDH activity.
Angiogenesis forms new vessels which connect with existing capillaries, accelerating the formation and growth of adipocytes [36]. By restricting MMP activity, the supply of oxygen and nutrients to adipose tissues are decreased, ultimately restricting adipocyte proliferation and differentiation. Thus, MMPs may serve as a valuable indicator of obesity restriction. Alexander [37] found that while 3T3-L1 preadipocytes differentiate, MMP-2 and MMP-9 are produced and released. This study, by treating peanut sprout ethanol extract with adipocytes, showed that there was a significant decrease in MMP-9 and MMP-2 activity, with MMP-9 activity being more significantly affected than MMP-2, resulting in a suppression of differentiation of adipocytes. Our previous in vitro study of resveratrol also showed that the activity of MMP-2 was decreased significantly by 84%, 70%, and 63% while MMP-9 activity was decreased significantly by 74%, 62%, and 39% with the increased resveratrol concentrations of 10, 20, and 40 µmol/L, respectively [34]. A in vivo study suggested that the elevated levels of MMP-9 were significantly attenuated in the resveratrol-treated mice treated with resveratrol for 7 days (50 mg/kg, gavage) as compared to the control mice [38]. A limitation of our study is that no positive control to demonstrate the responsiveness of in vitro preparations was involved in this study. Another limitation of this study is that another physiological components of peanut sprout ethanol extract (PSEE) except resveratrol were not determined. Therefore, further studies regarding other component of peanut sprouts and adipocyte proliferation and differentiation are needed.
In conclusion, this study suggests that the peanut sprouts have anti-obesity effects by inhibiting adipocyte proliferation and differentiation, by decreasing MMP-2 and MMP-9, which are important key regulator of adipocyte differentiation. The findings of the present study gives suggestive evidence for the possible usefulness of peanut sprout in the prevention and management of obesity.
Figures and Tables
 | Fig. 1
Effect of PSEE on cell growth in 3T3-L1 cells. 3T3-L1 cells were plated at a density of 1.5 × 104 cells/mL in 24 well plate with DMEM, supplemented with 10% FBS, 10 µg/mL insulin, 1 µmol/L Dex, and 0.5 mmol/L IBMX for 2 days. After differentiation induction, the monolayer was incubated in post-differentiation medium with 0, 10, 20, or 40 µg/mL PSEE. Viable cell numbers were estimated by the MTT assay. Each bar represents the mean ± SE. Comparison among different concentrations of PSEE that yielded significant differences (P < 0.05) are indicated by different letters above each bar. |
 | Fig. 2
Effect of PSEE on quantification of lipid content in 3T3-L1 cells. 3T3-L1 cells were plated at a density of 1.5 × 104 cells/mL in 24 well plate with DMEM supplemented with 10% FBS, 10 µg/mL insulin, 1 µmol/L Dex, and 0.5 mmol/L IBMX for 2 days. After differentiation induction, the monolayer was incubated in post-differentiation medium with 0, 10, 20, or 40 µg/mL PSEE. Lipid accumulation was estimated by the Oil-Red O staining. Each bar represents the mean ± SE. Comparison among different concentrations of PSEE that yielded significant differences (P < 0.05) are indicated by different letters above each bar. |
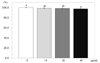 | Fig. 3
Effect of PSEE on triglyceride in 3T3-L1 cells. 3T3-L1 cells were plated at a density of 1.5 × 104 cells/mL in 24 well plate with DMEM supplemented with 10% FBS, 10 µg/mL insulin, 1 µmol/L Dex, and 0.5 mmol/L IBMX for 2 days. After differentiation induction, the monolayer was incubated in post-differentiation medium with 0, 10, 20, or 40 µg/mL PSEE. Triglyceride accumulation was estimated by the commercial triglyceride assay kit. Each bar represents the mean ± SE. Comparison among different concentrations of PSEE that yielded significant differences (P < 0.05) are indicated by different letters above each bar. |
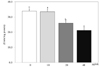 | Fig. 4
Effect of PSEE on GPDH activity in 3T3-L1 cells. 3T3-L1 cells were plated at a density of 1.5 × 104 cells/mL in 24 well plate with DMEM supplemented with 10% FBS, 10 µg/mL insulin, 1 µmol/L Dex, and 0.5 mmol/L IBMX for 2 days. After differentiation induction, the monolayer was incubated in post-differentiation medium with 0, 10, 20, or 40 µg/mL PSEE. GPDH activity were estimated by commercial GPDH activity assay kit. Each bar represents the mean ± SE. Comparison among different concentrations of PSEE that yielded significant differences (P < 0.05) are indicated by different letters above each bar. |
 | Fig. 5
Effect of PSEE on mRNA expression of transcription factors in 3T3-L1 cells. 3T3-L1 cells were plated at a density of 1.5 × 104 cells/mL in 24 well plate with DMEM supplemented with 10% FBS, 10 µg/mL insulin, 1 µmol/L Dex, and 0.5 mmol/L IBMX for 2 days. After differentiation induction, the monolayer was incubated in post-differentiation medium with 0, 10, 20, or 40 µg/mL PSEE. Chemiluminescent detection and quantitative analysis of western blots were performed for three independent experiments. The protein expression of C/EBP β (left) and C/EBP α (right) are shown above. Each bar represents the mean ± SE. Comparison among different concentrations of PSEE that yielded significant differences (P < 0.05) are indicated by different letters above each bar. |
 | Fig. 6
Effect of PSEE on MMPs activity in 3T3-L1 cells. 3T3-L1 cells were plated at a density of 1.5 × 104 cells/mL in 24 well plate with DMEM supplemented with 10% FBS, 10 µg/mL insulin, 1 µmol/L Dex, and 0.5 mmol/L IBMX for 2 days. After differentiation induction, the monolayer was incubated in post-differentiation medium with 0, 10, 20, or 40 µg/mL PSEE. Medium were collected and concentrated for zymography. Above photograph of MMPs bands, which were representative of three independent experiments, are shown. Under bars were quantitative analyses of zymography. Each bar represents the mean ± SE. Comparison among different concentrations of PSEE that yielded significant differences (P < 0.05) are indicated by different letters above each bar. |
References
1. Cornelius P, MacDougald OA, Lane MD. Regulation of adipocyte development. Annu Rev Nutr. 1994; 14:99–129.

2. Camp HS, Ren D, Leff T. Adipogenesis and fat-cell function in obesity and diabetes. Trends Mol Med. 2002; 8:442–447.

3. Rosen ED, Walkey CJ, Puigserver P, Spiegelman BM. Transcriptional regulation of adipogenesis. Genes Dev. 2000; 14:1293–1307.

4. Bouloumié A, Sengenès C, Portolan G, Galitzky J, Lafontan M. Adipocyte produces matrix metalloproteinases 2 and 9: involvement in adipose differentiation. Diabetes. 2001; 50:2080–2086.
5. Bhat KP, Pezzuto JM. Cancer chemopreventive activity of resveratrol. Ann N Y Acad Sci. 2002; 957:210–229.

6. de la Lastra CA, Villegas I. Resveratrol as an antioxidant and pro-oxidant agent: mechanisms and clinical implications. Biochem Soc Trans. 2007; 35:1156–1160.

7. Das S, Alagappan VK, Bagchi D, Sharma HS, Maulik N, Das DK. Coordinated induction of iNOS-VEGF-KDR-eNOS after resveratrol consumption: a potential mechanism for resveratrol preconditioning of the heart. Vascul Pharmacol. 2005; 42:281–289.
8. Kopp P. Resveratrol, a phytoestrogen found in red wine. A possible explanation for the conundrum of the 'French paradox'? Eur J Endocrinol. 1998; 138:619–620.

9. Hardie DG, Pan DA. Regulation of fatty acid synthesis and oxidation by the AMP-activated protein kinase. Biochem Soc Trans. 2002; 30:1064–1070.

10. Szkudelska K, Nogowski L, Szkudelski T. Resveratrol, a naturally occurring diphenolic compound, affects lipogenesis, lipolysis and the antilipolytic action of insulin in isolated rat adipocytes. J Steroid Biochem Mol Biol. 2009; 113:17–24.

11. Dal-Pan A, Blanc S, Aujard F. Resveratrol suppresses body mass gain in a seasonal non-human primate model of obesity. BMC Physiol. 2010; 10:11.

12. Gu X, Creasy L, Kester A, Zeece M. Capillary electrophoretic determination of resveratrol in wines. J Agric Food Chem. 1999; 47:3223–3227.

13. Wang KH, Lai YH, Chang JC, Ko TF, Shyu SL, Chiou RY. Germination of peanut kernels to enhance resveratrol biosynthesis and prepare sprouts as a functional vegetable. J Agric Food Chem. 2005; 53:242–246.

14. Kang HI, Kim JY, Park KW, Kang JS, Choi MR, Moon KD, Seo KI. Resveratrol content and nutritional components in peanut sprouts. Korean J Food Preserv. 2010; 17:384–390.
15. Kang HI, Kim JY, Kwon SJ, Park KW, Kang JS, Seo KI. Antioxidative effects of peanut sprout extracts. J Korean Soc Food Sci Nutr. 2010; 39:941–946.

16. Lin BS, Lien TF, Chao MR, Lai TY, Chang JC, Chou SJ, Liao HF, Chiou RY. Toxicological and nutraceutical assessments of peanut sprouts as daily supplements to feed Sprague-Dawley rats for 18 weeks. J Sci Food Agric. 2008; 88:2201–2207.

17. Green H, Kehinde O. An established preadipose cell line and its differentiation in culture. II. Factors affecting the adipose conversion. Cell. 1975; 5:19–27.

18. Lee MS, Kim CT, Kim CJ, Cho YJ, Kim Y. Effects of Portulaca oleracea L. extract on lipolysis and hormone sensitive lipase (HSL) gene expression in 3T3-L1 adipocytes. Korean J Nutr. 2006; 39:742–747.
19. Kim MJ, Kim Y, Chung JH, Kim JW, Kim HK. The effect of caffeine on 3T3-L1 adipocyte differentiation: a nutrigenomical approach. Korean J Nutr. 2005; 38:649–655.
20. Chon JW, Sung JH, Hwang EJ, Park YK. Chlorella methanol extract reduces lipid accumulation in and increases the number of apoptotic 3T3-L1 cells. Ann N Y Acad Sci. 2009; 1171:183–189.

21. Tomiyama K, Nakata H, Sasa H, Arimura S, Nishio E, Watanabe Y. Wortmannin, a specific phosphatidylinositol 3-kinase inhibitor, inhibits adipocytic differentiation of 3T3-L1 cells. Biochem Biophys Res Commun. 1995; 212:263–269.

22. Ramírez-Zacarías JL, Castro-Muñozledo F, Kuri-Harcuch W. Quantitation of adipose conversion and triglycerides by staining intracytoplasmic lipids with Oil red O. Histochemistry. 1992; 97:493–497.

23. Na MH, Seo EY, Kim WK. Effects of α-lipoic acid on cell proliferation and apoptosis in MDA-MB-231 human breast cells. Nutr Res Pract. 2009; 3:265–271.

24. Kwon SY, Kang KJ. The effect of conjugated linoleic acid isomers on the cell proliferation, apoptosis and expressions of uncoupling protein (UCP) genes during differentiation of 3T3-L1 preadipocytes. Korean J Nutr. 2004; 37:533–539.
25. Kyoya T, Ishida A, Nakashima K, Nakajima I, Toyoda A, Nakamura Y, Katsumata M. The effects of concentrations of lysine in media on differentiation of 3T3-L1 preadipocytes. Anim Sci J. 2011; 82:565–570.

26. Olofsson LE, Orho-Melander M, William-Olsson L, Sjöholm K, Sjöström L, Groop L, Carlsson B, Carlsson LM, Olsson B. CCAAT/enhancer binding protein alpha (C/EBPalpha) in adipose tissue regulates genes in lipid and glucose metabolism and a genetic variation in C/EBPalpha is associated with serum levels of triglycerides. J Clin Endocrinol Metab. 2008; 93:4880–4886.

27. Cho KJ, Moon HE, Moini H, Packer L, Yoon DY, Chung AS. Alpha-lipoic acid inhibits adipocyte differentiation by regulating pro-adipogenic transcription factors via mitogen-activated protein kinase pathways. J Biol Chem. 2003; 278:34823–34833.

28. Hong MK, Cho KY, Oh SJ, Kim KM, Yu SJ, Jung SS. Implications of the activation of matrix metalloproteinase-2 (MMP-2) on the metastasis in breast cancer. J Korean Surg Soc. 2002; 62:18–25.

29. Rural Development Administration, National Institute of Crop Science [Internet]. Suwon: Rural Development Administration;2010. cited 2010 October 14. Available from: http://rda.korea.kr/gonews.
30. Shin HD. Anti-obesity effect of vegetable sprouts in 3T3-L1 adipocytes and rats [master's thesis]. Gwangju: Chosun University;2007.
31. Wise LS, Green H. Participation of one isozyme of cytosolic glycerophosphate dehydrogenase in the adipose conversion of 3T3 cells. J Biol Chem. 1979; 254:273–275.

32. Rayalam S, Yang JY, Ambati S, Della-Fera MA, Baile CA. Resveratrol induces apoptosis and inhibits adipogenesis in 3T3-L1 adipocytes. Phytother Res. 2008; 22:1367–1371.

33. Zhang XH, Huang B, Choi SK, Seo JS. Anti-obesity effect of resveratrol-amplified grape skin extracts on 3T3-L1 adipocytes differentiation. Nutr Res Pract. 2012; 6:286–293.

34. Kang NE, Ha AW, Kim JY, Kim WK. Resveratrol inhibits the protein expression of transcription factors related adipocyte differentiation and the activity of matrix metalloproteinase in mouse fibroblast 3T3-L1 preadipocytes. Nutr Res Pract. 2012; 6:499–504.

35. Park HJ, Yang JY, Ambati S, Della-Fera MA, Hausman DB, Rayalam S, Baile CA. Combined effects of genistein, quercetin, and resveratrol in human and 3T3-L1 adipocytes. J Med Food. 2008; 11:773–783.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download